Search found 52 matches
- Sun Mar 18, 2018 10:04 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Spontaneity in a cell reaction
- Replies: 4
- Views: 863
Re: Spontaneity in a cell reaction
Based on the equation Matt gave, if Ecell is positive, Delta G is negative = spontaneous. If Ecell is negative, Delta G is positive = nonspontaneous.
- Sun Mar 18, 2018 9:46 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Pt electrode
- Replies: 2
- Views: 464
Re: Pt electrode
You also add Pt when there is a solid that cannot conduct electricity (e.g. halogen)
- Fri Mar 16, 2018 10:20 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Question from test 1
- Replies: 1
- Views: 401
Re: Question from test 1
a. Yes, there is a phase change occurring because liquefied propane was released as gaseous propane vapors.
b. Yes, the temperature remained constant, meaning the surroundings had to supplement heat to keep the temperature from changing.
b. Yes, the temperature remained constant, meaning the surroundings had to supplement heat to keep the temperature from changing.
- Fri Mar 16, 2018 10:16 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Midterm question 6a
- Replies: 5
- Views: 908
Re: Midterm question 6a
They were both correct because complex molecules have more entropy than less complex molecules and gas has more entropy than liquid.
- Fri Mar 16, 2018 10:14 pm
- Forum: *Nucleophiles
- Topic: nucleophile?
- Replies: 4
- Views: 1472
Re: nucleophile?
The nucleophile attacks a positively charged, for example carbon, and in the process, substitutes with the leaving group.
- Fri Mar 16, 2018 10:01 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: sign of k
- Replies: 3
- Views: 771
Re: sign of k
No, rate constants can never be negative.
- Fri Mar 16, 2018 5:03 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Test #2 question 4
- Replies: 4
- Views: 530
Re: Test #2 question 4
When you flip the sign because you flip the equation, you must do Ecell = Ecathode + Eanode.
It is also helpful to know that for galvanic cells, the Ecell must always be positive.
It is also helpful to know that for galvanic cells, the Ecell must always be positive.
- Thu Mar 15, 2018 3:39 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Coefficients in Rate Law
- Replies: 2
- Views: 426
Re: Coefficients in Rate Law
Just adding on that with reaction mechanisms, the rate law will sometimes have the same exponents as the coefficients in the chemical equation by mere coincidence. We cannot assume that the coefficients will be the exponents. We must look at the elementary steps.
- Thu Mar 15, 2018 1:29 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: catalysts
- Replies: 5
- Views: 724
Re: catalysts
Catalysts can be appear in the overall rate law, but sometimes they are cancelled out (e.g. consumed in the fast step before and produced in the slow step) and therefore would not appear in the rate law.
- Thu Mar 15, 2018 1:26 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Which equilibrium equation to use
- Replies: 2
- Views: 374
Re: Which equilibrium equation to use
If a fast step is before a slow step, we can assume the fast step is in equilibrium. I believe Lavelle called it pseudo equilibrium.
We substitute equilibrium constant K to get rid of any intermediates because intermediates cannot be in the rate law.
We substitute equilibrium constant K to get rid of any intermediates because intermediates cannot be in the rate law.
- Thu Mar 15, 2018 1:13 pm
- Forum: General Rate Laws
- Topic: Test #3 Q5
- Replies: 3
- Views: 494
Re: Test #3 Q5
Since both reactants are first order, the rate law would be rate law = k[CHCL3][CL2]. Because you are given the instantaneous rate of reaction, 2.54 x 10^/2 mol/L and the concentrations can be solved for, you just plug those numbers into the rate law equation to find k. To find the concentration of ...
- Tue Mar 06, 2018 10:18 pm
- Forum: General Rate Laws
- Topic: 15.5
- Replies: 3
- Views: 437
Re: 15.5
Someone please correct me if I am wrong, but do we multiply the unique average rate by the coefficients (for e.g. oxygen) to find its rate because the unique average rate already took into account its coefficients and now we just have to multiply it back?
- Tue Mar 06, 2018 10:14 pm
- Forum: General Rate Laws
- Topic: 15.5
- Replies: 3
- Views: 437
Re: 15.5
I thought the unique average rate law took into account the stoichiometric coefficients so it's not necessarily just one mole
- Tue Mar 06, 2018 10:06 pm
- Forum: First Order Reactions
- Topic: 15.23 c
- Replies: 1
- Views: 272
Re: 15.23 c
The equation given is 2A --> B + C So there is a decrease in A because it is making B (an increase in B) If we know the increase in B, we can use the coefficients from the equation to convert moles of B back to moles of A. The number we get from the conversion is how much A was used to make B. So if...
- Tue Mar 06, 2018 10:00 pm
- Forum: General Rate Laws
- Topic: Proper units
- Replies: 7
- Views: 832
Re: Proper units
The textbook tends to write the answers in moles
- Tue Mar 06, 2018 9:55 pm
- Forum: General Rate Laws
- Topic: 15.5
- Replies: 3
- Views: 437
15.5
Why do we multiply the unique rate by the coefficient of oxygen to find its rate and multiply the unique rate by the coefficient of water to find its rate? Why don't we divide the unique rate by the coefficient?
- Wed Feb 28, 2018 4:32 pm
- Forum: General Rate Laws
- Topic: Negative 1/a
- Replies: 8
- Views: 1115
Re: Negative 1/a
Because the reactant is being consumed, the overall concentration of the reactant is decreasing so there is a negative rate of change. However, if the question asked at what rate was A being consumed, then it will be a positive answer. Just be careful about how the question is worded! Can you pleas...
- Wed Feb 28, 2018 4:23 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Test 3
- Replies: 6
- Views: 875
Re: Test 3
Yes, it only goes to #39
- Wed Feb 28, 2018 4:20 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Textbook Problems 15.3 and 15.5
- Replies: 7
- Views: 1085
Re: Textbook Problems 15.3 and 15.5
So when you are calculating the rate of reaction that is not the unique rate of reaction, you multiply the concentration by the coefficient?
- Wed Feb 28, 2018 4:16 pm
- Forum: General Rate Laws
- Topic: 15.3 C
- Replies: 8
- Views: 1619
Re: 15.3 C
The unique reaction rate takes into account the coefficients of the chemical equation.
- Wed Feb 28, 2018 4:12 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: 15.3 c
- Replies: 2
- Views: 404
Re: 15.3 c
The unique rate of reaction is the general rate of (for example) 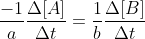
when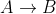
This rate of reaction takes into account the coefficients of the reaction.
when
This rate of reaction takes into account the coefficients of the reaction.
- Tue Feb 27, 2018 10:51 pm
- Forum: General Rate Laws
- Topic: 15. 17 [ENDORSED]
- Replies: 2
- Views: 431
15. 17 [ENDORSED]
How do you know when a reactant is independent of the rate?
- Thu Feb 22, 2018 7:40 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagram Order for Same Phases
- Replies: 4
- Views: 612
Re: Cell Diagram Order for Same Phases
I put it in order of the reactants, products
I am not sure if this is completely right but I have gotten the same answers so far
I am not sure if this is completely right but I have gotten the same answers so far
- Wed Feb 21, 2018 12:45 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Oxidizing vs. Reducing agent
- Replies: 1
- Views: 317
Re: Oxidizing vs. Reducing agent
The oxidizing agent is the substance being reduced.
The reducing agent is the substance being oxidized.
When the cell potential is more positive, it has a stronger oxidizing agent
When the cell potential is more negative, it has a stronger reducing agent
The reducing agent is the substance being oxidized.
When the cell potential is more positive, it has a stronger oxidizing agent
When the cell potential is more negative, it has a stronger reducing agent
- Wed Feb 21, 2018 12:34 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagrams
- Replies: 3
- Views: 437
Re: Cell Diagrams
The commas mean that they are in the same phase.
" | " means that they are in different phases
"||" is the salt bridge that separates the anode from the cathode
" | " means that they are in different phases
"||" is the salt bridge that separates the anode from the cathode
- Tue Feb 20, 2018 6:55 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: H+
- Replies: 2
- Views: 510
Re: H+
When you need to balance out the oxygens, you add H20 to other side. Then to balance the H's from the H20, you add H+ to the side of the oxygens so everything balances out.
- Tue Feb 20, 2018 6:53 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagram Structure
- Replies: 3
- Views: 405
Re: Cell Diagram Structure
You use one | to represent different phases in contact with each other and two || to indicate the salt bridge.
- Tue Feb 20, 2018 6:52 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagram
- Replies: 2
- Views: 288
Re: Cell Diagram
I think we use Pt when they are no other solids to transfer electrons.
- Wed Feb 14, 2018 11:55 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Temperature Relation
- Replies: 2
- Views: 389
Temperature Relation
Can someone please explain the relationship between temperature and internal energy ?
Also, the relationship between temperature and entropy?
Thanks
Also, the relationship between temperature and entropy?
Thanks
- Tue Feb 13, 2018 11:01 pm
- Forum: Calculating Work of Expansion
- Topic: Formulas for monatomic vs diatomic gases
- Replies: 2
- Views: 547
Formulas for monatomic vs diatomic gases
What formulas do you use when it is a) monatomic gas b) diatomic gas?
- Tue Feb 13, 2018 4:52 pm
- Forum: Van't Hoff Equation
- Topic: Vant Hoff Equation
- Replies: 1
- Views: 303
Vant Hoff Equation
How would you use the Vant Hoff Equation when there are two different temperatures given?
Do you solve for one temperature first and then the other and add them?
Do you solve for one temperature first and then the other and add them?
- Tue Feb 13, 2018 3:53 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Delta H at constant pressure
- Replies: 2
- Views: 365
Re: Delta H at constant pressure
At constant pressure delta H = q, which means q would have to be 0.
q is 0 when it is an adiabatic reaction.
q is 0 when it is an adiabatic reaction.
- Tue Feb 13, 2018 3:50 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Constant Volume
- Replies: 2
- Views: 231
Re: Constant Volume
When w = 0, delta U = q.
q is only equal to delta H when it is q is at constant pressure.
q is only equal to delta H when it is q is at constant pressure.
- Sat Feb 10, 2018 9:55 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Entropy
- Replies: 7
- Views: 717
Re: Entropy
S°m is molar entropy.
S°f is standard entropy of formation.
S°r is standard reaction entropy.
S°f is standard entropy of formation.
S°r is standard reaction entropy.
- Sat Feb 10, 2018 9:51 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Exothermic/Endothermic [ENDORSED]
- Replies: 3
- Views: 739
Re: Exothermic/Endothermic [ENDORSED]
I think of catabolic reactions breaking down things, releasing energy = exothermic and anabolic reactions forming things, requiring energy = endothermic.
These reactions tend to go hand in hand with catabolic reactions supplying the energy for anabolic reactions.
These reactions tend to go hand in hand with catabolic reactions supplying the energy for anabolic reactions.
- Thu Feb 08, 2018 8:58 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Spontaneity
- Replies: 9
- Views: 1287
Re: Spontaneity
Negative delta G means the reaction is spontaneous.
- Sat Feb 03, 2018 2:00 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Adiabatic [ENDORSED]
- Replies: 4
- Views: 507
Re: Adiabatic [ENDORSED]
When a reaction is adiabatic, q=0
- Sat Feb 03, 2018 1:57 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Question 3b on the test
- Replies: 4
- Views: 661
Re: Question 3b on the test
The isothermal reaction tells us that internal energy is equal to 0, which means q = -w. Since the temperature stayed constant while there was work being done, we know that there was a heat transfer from the surroundings into the system to maintain the temperature.
- Sat Feb 03, 2018 1:51 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: What does it mean for a reaction to be "thermodynamically favored?"
- Replies: 6
- Views: 31786
Re: What does it mean for a reaction to be "thermodynamically favored?"
Reactions that do not require energy are seen as more favorable. Since exothermic reactions release energy and endothermic reactions require energy, exothermic reactions are more favorable.
- Thu Jan 25, 2018 9:52 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Internal Energy
- Replies: 5
- Views: 525
Re: Internal Energy
If it was delta P instead of P in the equation, then pressure would equal 0 when it is constant.
- Thu Jan 25, 2018 9:47 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: DeltaU and DeltaH
- Replies: 4
- Views: 923
Re: DeltaU and DeltaH
Delta U = Delta H when there is constant pressure and constant volume. Remember that Delta U = q +w. Delta H = q when there is constant pressure. w= -P Delta V. So when volume is constant, Delta V = 0. Therefore when there is constant pressure and volume, you can substitute q & w and get delta U...
- Tue Jan 23, 2018 10:28 pm
- Forum: Phase Changes & Related Calculations
- Topic: Phase Changes Equations
- Replies: 4
- Views: 586
Phase Changes Equations
Delta H fusion = Hm (liquid) - Hm (solid) as mentioned in the book and in lecture. What does Hm stand for? Are we going to have to know how to solve for delta H fusion or for the other delta H phase changes?
- Tue Jan 23, 2018 9:05 pm
- Forum: Phase Changes & Related Calculations
- Topic: Phase Changes and Energy
- Replies: 2
- Views: 345
Re: Phase Changes and Energy
Melting, vaporization, and sublimation all require energy (endothermic).
Condensation releases energy.
Condensation releases energy.
- Tue Jan 23, 2018 9:01 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Units
- Replies: 2
- Views: 293
Re: Units
I would just look at the units in your equation that you are trying to solve and see if the moles cancel out. If not, then delta H is kJ/mol. If the moles cancel out, then delta H is kJ.
- Tue Jan 23, 2018 8:58 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Exothermic
- Replies: 7
- Views: 893
Re: Exothermic
An exothermic reaction releases energy, which means that heat is also being released, causing a rise of temperature.
- Tue Jan 23, 2018 8:53 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Reaction Enthalpies vs other methods
- Replies: 2
- Views: 312
Re: Reaction Enthalpies vs other methods
The 3 methods are just specific types of reaction enthalpies.
- Fri Jan 19, 2018 2:44 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.45 question
- Replies: 4
- Views: 459
Re: 8.45 question
I am also confused because in my notes I have that endothermic reactions absorbs and requires energy so breaking bonds would be endothermic. Exothermic releases and in my notes I put forming bonds would be exothermic but why would you release energy when you form bonds?
- Fri Jan 19, 2018 2:32 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Homework 87
- Replies: 2
- Views: 191
Re: Homework 87
When a reaction is endothermic, delta H is positive. Reactions that are endothermic include vaporization, melting/fusion,and sublimation because heat is being absorbed. When a reaction is exothermic, delta H is negative. A reaction that exothermic is combustion because heat is released.
- Fri Jan 19, 2018 2:26 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Calorimeter vs. Bomb Calorimeter
- Replies: 2
- Views: 311
Re: Calorimeter vs. Bomb Calorimeter
A normal calorimeter is for constant pressure and a bomb calorimeter is for constant volume.
- Sun Jan 14, 2018 5:57 pm
- Forum: Phase Changes & Related Calculations
- Topic: Isolated vs Closed Systems
- Replies: 2
- Views: 209
Re: Isolated vs Closed Systems
An isolated system does not come in contact with its surroundings (heat stays in thermos).
A closed system has a fixed amount of matter and can exchange energy with its surroundings (the ice pack is used on injuries to exchange energy).
A closed system has a fixed amount of matter and can exchange energy with its surroundings (the ice pack is used on injuries to exchange energy).
- Sun Jan 14, 2018 3:56 pm
- Forum: Phase Changes & Related Calculations
- Topic: Steam Burning
- Replies: 7
- Views: 948
Re: Steam Burning
Steam burns more because of the phase change from liquid to vapor. Water boils at 100 degrees Celsius and during the phase change of vaporization, heat continues to increase. So when the boiling water has been turned into steam, a lot more heat had been supplied and is the reason why steam burns more.
- Wed Jan 10, 2018 7:13 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Heat Capacity vs. Specific Heat Capacity
- Replies: 6
- Views: 1088
Re: Heat Capacity vs. Specific Heat Capacity
Heat capacity is the amount of heat over how much the temperature increased; it's a ratio. Whereas specific heat capacity is the heat capacity divided by the mass of the sample. Molar heat capacity is similar to specific heat capacity but instead of dividing the heat capacity by the mass, you're div...

