Search found 43 matches
- Sun Mar 18, 2018 9:07 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: S and pressure
- Replies: 3
- Views: 719
S and pressure
Will S increase if pressure increases or decreases?
- Sun Mar 18, 2018 9:05 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: w
- Replies: 2
- Views: 702
w
just to verify: w is the number of orientation (# of ways to arrange the atoms in a molecule) to the power of however many molecules are involved?
- Sun Mar 18, 2018 9:03 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: metal dissolved
- Replies: 2
- Views: 495
metal dissolved
what's the best way to distinguish if a metal will dissolve in a solution?
- Sun Mar 18, 2018 9:02 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: work, cell potential, and free energy
- Replies: 1
- Views: 401
work, cell potential, and free energy
what is the relationship between work, cell potential, and free energy?
- Sun Mar 18, 2018 9:00 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: maximum potential
- Replies: 1
- Views: 410
maximum potential
how does emf represent the maximum potential difference?
- Sun Mar 18, 2018 8:59 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: rate of change
- Replies: 4
- Views: 792
rate of change
how does the rate of change of one species in a reaction relate to another species?
- Sun Mar 18, 2018 8:56 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: arrhenius
- Replies: 1
- Views: 414
arrhenius
where does the equation k2/k1=H/R(1/T -1/T) come from? I can't figure out the derivation.
- Sun Mar 18, 2018 8:50 pm
- Forum: General Rate Laws
- Topic: rate proportions
- Replies: 2
- Views: 662
rate proportions
what does a rate proportion depend on? for example what will happen to a rate if the amount of a molecule doubles or halves?
- Sun Mar 18, 2018 8:42 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: change in volume and then temperature
- Replies: 1
- Views: 391
change in volume and then temperature
if there is a change in volume and then change in temperature would finding each and then adding both provide the overall entropy value?
- Sun Mar 18, 2018 8:38 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: mechanism order
- Replies: 1
- Views: 421
mechanism order
if a slow step is the first step and the fast step is the second in a mechanism, will the rate just be based on the first step or would you have to remove the intermediate and use k for a substitute in the second step?
- Sun Mar 18, 2018 8:36 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: catalysts
- Replies: 3
- Views: 852
catalysts
how do you identify a catalyst in a two step reaction?
- Sun Mar 18, 2018 8:34 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: cell diagram placement
- Replies: 1
- Views: 419
cell diagram placement
I know that the left side of the salt bridge in a cell diagram represents the anode and the right side the cathode. The single line differentiates reaction/product. But sometimes it isn't written in that way so I just want to know how to correctly place the factors in a cell diagram?
- Sun Mar 18, 2018 8:30 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: mechanism differentiation
- Replies: 2
- Views: 577
mechanism differentiation
how do you differentiate between two mechanism using rate laws?
- Sun Mar 18, 2018 8:28 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: microstate and expansion
- Replies: 1
- Views: 396
microstate and expansion
So if a gas expands the microstate of a molecule will increase?
- Sun Mar 18, 2018 8:27 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: n: negative or positive
- Replies: 2
- Views: 1094
n: negative or positive
So when you plug in n for the electron it corresponds to in equations will it always be negative?
- Sun Mar 18, 2018 8:21 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Conductor
- Replies: 1
- Views: 357
Conductor
When do you know when to use a conductor? And what side you would add it on a cell diagram?
- Sat Mar 17, 2018 7:20 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Solve for Q
- Replies: 2
- Views: 489
Solve for Q
How do you solve for Q when you aren't given E?
asking from test 2 #8b... or do you just divide the moralities given? if so i got 1.5*10^5 is that correct?
asking from test 2 #8b... or do you just divide the moralities given? if so i got 1.5*10^5 is that correct?
- Sat Mar 17, 2018 6:27 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Test 2 #3
- Replies: 2
- Views: 497
Test 2 #3
I re-did this problem and want to make sure i got the right answer: K=1.6 10^15 and therefore it is spontaneous.
- Sat Mar 17, 2018 6:01 am
- Forum: Balancing Redox Reactions
- Topic: Test 2
- Replies: 2
- Views: 449
Test 2
I just want to double check my answer for #1 on the test: a) i got the oxidation state for C will be +4 and b) the reduced species is oxygen
- Sat Mar 17, 2018 5:40 am
- Forum: Balancing Redox Reactions
- Topic: reducing and oxidizing agents
- Replies: 2
- Views: 436
reducing and oxidizing agents
Why are reducing and oxidizing agents the opposites of reduction and oxidation?
- Sat Mar 17, 2018 1:46 am
- Forum: Phase Changes & Related Calculations
- Topic: Sign for compression vs expansion
- Replies: 4
- Views: 1140
Sign for compression vs expansion
When using the equation for w=-P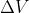 is it true that for compression the answer will be positive and for expansion it will be negative?
is it true that for compression the answer will be positive and for expansion it will be negative?
- Tue Mar 13, 2018 6:56 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Standard Hydrogen Electrode
- Replies: 1
- Views: 249
Standard Hydrogen Electrode
What is the best way to identify a cathode and an anode in a cell diagram? I thought I understood it but I got confused while reading the book. On pg 578 it says that the cell potential will be attributed to the copper electrode because the standard hydrogen electrode is always zero, but for the zin...
- Fri Mar 09, 2018 6:49 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation Number
- Replies: 4
- Views: 544
Oxidation Number
The example of the textbook 14.1 says that Mn has an oxidation number of +7, where does this number come from?
- Tue Feb 13, 2018 6:38 pm
- Forum: Calculating Work of Expansion
- Topic: -w vs +w
- Replies: 2
- Views: 407
-w vs +w
Just to clarify: if work is positive that means expansion and if it is negative it means compression?
- Mon Jan 22, 2018 9:14 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Reaction Sequences
- Replies: 1
- Views: 228
Reaction Sequences
Is there an easy, quick way to calculate the overall reaction enthalpy from the enthalpies of the reactions in a reaction sequence? I understand the steps but it takes me a while so I'm wondering if there is a quicker way or is it mostly trial and error.
- Sun Jan 21, 2018 1:05 am
- Forum: Phase Changes & Related Calculations
- Topic: Equations to know
- Replies: 6
- Views: 457
Equations to know
Are ALL the formulas we need to know gonna be in the "Constants and Formulas " sheet provided for us or are there any that aren't on there nut we should know?
- Sat Jan 20, 2018 11:34 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.37
- Replies: 1
- Views: 195
8.37
Why is the enthalpy of vaporization for this problem 4.76 kJ of heat/0.579 mol (from solution manual)?
- Sat Jan 20, 2018 5:51 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: 8.5
- Replies: 3
- Views: 431
8.5
In the question, it says 340 kJ of work is compressed so shouldn't it be negative (because it is being compressed) when we add it to 524 kJ of heat? (so instead of 864 kJ it is 184 kJ?)
- Sat Jan 20, 2018 4:29 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Example 8.7
- Replies: 1
- Views: 170
Example 8.7
Why is the equation to figure the change in enthalpy  =(2mol)*q/n ? I understand the 2 mol comes from benzene but why do we have to do q/n?
=(2mol)*q/n ? I understand the 2 mol comes from benzene but why do we have to do q/n?
- Tue Jan 16, 2018 1:04 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: K vs C
- Replies: 6
- Views: 571
Re: K vs C
SO just to be sure because i had the same question concerning K vs C: you can use K and C interchangeably without affecting the answer?
- Tue Nov 28, 2017 8:43 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: ICE Box
- Replies: 3
- Views: 639
ICE Box
When determining the change in pressure, how do you know whether the "x" will be positive or negative? What determines this?
- Tue Nov 28, 2017 8:42 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: ICE Box
- Replies: 1
- Views: 328
ICE Box
how do you get the sum of the first two reactions to equal the third reaction? asking from example in Chapter 11 pg 439.
Also why do we multiply the second reaction by a factor of 2?
Also why do we multiply the second reaction by a factor of 2?
- Wed Nov 22, 2017 2:50 am
- Forum: Photoelectric Effect
- Topic: Higher Frequency and Kinetic Energy [ENDORSED]
- Replies: 5
- Views: 1123
Higher Frequency and Kinetic Energy [ENDORSED]
Higher frequency light always emits electrons with higher: kinetic energy or low kinetic energy?
Asking from Midterm Q3 D
Asking from Midterm Q3 D
- Wed Nov 22, 2017 2:38 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K and Kc
- Replies: 7
- Views: 1085
K and Kc
What is the difference between using K and Kc?
- Wed Nov 22, 2017 2:37 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: "Phase"
- Replies: 5
- Views: 575
"Phase"
what does it mean for reactants and products to be in "phase"? What is a phase?
- Wed Nov 22, 2017 2:34 am
- Forum: Dipole Moments
- Topic: Dipole Moments
- Replies: 2
- Views: 416
Dipole Moments
How can you determine which element will affect dipole moments? Or is it depending on the compound? In other words, is determining dipole moments dependent on what the element is in a compound or the structure of a compound? I'm having a hard time grasping dipole moments.
- Wed Nov 22, 2017 1:18 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Chemical Equilibrium
- Replies: 2
- Views: 377
Chemical Equilibrium
When writing the equilibrium constant for a reaction, why do we exclude solids, solvents, and liquids? Are those the only solutions we exclude when writing the equilibrium constant expression?
- Mon Nov 20, 2017 8:06 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Chapter 11 Reading
- Replies: 1
- Views: 286
Chapter 11 Reading
Can anyone tell me what  means or refers to in the reading of chapter 11?
means or refers to in the reading of chapter 11?
- Mon Nov 13, 2017 10:18 pm
- Forum: Limiting Reactant Calculations
- Topic: Limiting Reactant vs Theoretical Yield
- Replies: 3
- Views: 1791
Limiting Reactant vs Theoretical Yield
What is the difference between a limiting reactant and a theoretical yield? Or are they the same?
- Mon Nov 13, 2017 10:09 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Cation & Anion Polarity
- Replies: 3
- Views: 2518
Cation & Anion Polarity
Does the polarizing power of a cation or anion depend on its size? i.e. the smallest cation or largest anion is the most polarizable?
- Mon Nov 13, 2017 9:57 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Ionization energy
- Replies: 2
- Views: 792
Ionization energy
In ranking C N O F in order of increasing ionization energy, the answer is C<O<N<F; but why is Oxygen more ionic than Nitrogen?
- Mon Nov 13, 2017 9:52 pm
- Forum: Electronegativity
- Topic: Electroegativity
- Replies: 9
- Views: 1295
Electroegativity
How can you tell whether one compound is more electronegative than another one without using the electronegativity trend?
- Mon Nov 13, 2017 9:22 pm
- Forum: Electronegativity
- Topic: Isoelectronic [ENDORSED]
- Replies: 11
- Views: 1542
Isoelectronic [ENDORSED]
What does it mean for elements to be isoelectronic?

