Search found 34 matches
- Sun Mar 11, 2018 9:25 pm
- Forum: *Enzyme Kinetics
- Topic: Enzymes
- Replies: 8
- Views: 1715
Re: Enzymes
Right, enzymes are a type of catalyst that lower activation energy so the rate is faster.
- Sun Mar 11, 2018 8:40 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Steps
- Replies: 3
- Views: 469
Steps
How do we determine which step is a slow step and which step is a fast step in a reaction?
- Sun Mar 11, 2018 8:35 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Slowest Step
- Replies: 3
- Views: 514
Re: Slowest Step
The slowest step is what hinders the reaction, so the rate of reaction can only go as quickly as the slowest step.
- Tue Feb 27, 2018 7:09 pm
- Forum: General Rate Laws
- Topic: Orders and units
- Replies: 3
- Views: 561
Orders and units
Why is that each different order type (zero, first, second) has different units? Can someone explain it please?
- Sat Feb 24, 2018 4:50 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: n
- Replies: 8
- Views: 902
n
what is "n" in terms of kinetics?
- Sat Feb 24, 2018 4:43 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: kinetics
- Replies: 5
- Views: 752
kinetics
How are kinetics and thermodynamics related?
- Sun Feb 18, 2018 11:41 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: The Daniell Cell
- Replies: 4
- Views: 583
Re: The Daniell Cell
For galvanic cells, we mainly need to focus on what constitutes as a galvanic cell and the direction of electron flow.
- Sun Feb 18, 2018 11:17 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: redox
- Replies: 7
- Views: 1224
redox
So in an oxidation-reduction reaction, which is the reducing and which is the oxidizing agent?
- Sun Feb 18, 2018 11:13 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Salt Bridge
- Replies: 4
- Views: 438
Re: Salt Bridge
The salt bridge is used as a link between the half cells of the redox and oxidation reactions. The salt bridge helps maintain the charge as electrons flow from the anode to the cathode.
- Sun Feb 11, 2018 3:10 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Pt (s) [ENDORSED]
- Replies: 4
- Views: 641
Re: Pt (s) [ENDORSED]
If not stated, then it is assumed that Pt(s) is the metal used for both the anode and cathode.
- Sun Feb 11, 2018 3:08 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Standard Cell Potential
- Replies: 4
- Views: 1117
Re: Standard Cell Potential
Cell potential is the difference between a redox reaction and an oxidation reaction. Redox potential only consists of the reduction from the half reaction in the cathode.
- Sun Feb 11, 2018 2:51 pm
- Forum: Balancing Redox Reactions
- Topic: nernst eq'n
- Replies: 2
- Views: 387
nernst eq'n
So I was looking over the nernst equation, and apparently I forgot to write what the terms represent, I know most of them by common knowledge, but I frgot what the "n" means. It's not moles right?
- Sun Feb 04, 2018 1:51 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Delta G not
- Replies: 5
- Views: 791
Re: Delta G not
It's just a difference of values, the concept of 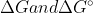 are still the same. I think that it just depends of the conditions of the reaction. I'm not entirely sure though, that's a good question.
are still the same. I think that it just depends of the conditions of the reaction. I'm not entirely sure though, that's a good question.
- Sun Feb 04, 2018 1:37 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Energy Free to do Useful Work
- Replies: 2
- Views: 414
Re: Energy Free to do Useful Work
Gibbs free energy is the energy that's left at the end of a reaction. This energy is still considered "useful work" because it's all the available energy that can be used for a specific purpose. Take the process of cellular respiration for example. By using oxygen and glucose, we can creat...
- Sun Feb 04, 2018 1:03 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Equilibrium Value K
- Replies: 3
- Views: 603
Re: Equilibrium Value K
At equilibrium, K=1; this would represent a reaction at equilibrium, since both the reactants nor products are favored. \Delta G is zero at equilibrium due to the fact that in the equation: \Delta H-T*\Delta S if both enthalpy and entropy equal zero, then there is no change, and \Delta G would be 0.
- Sun Jan 28, 2018 3:13 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Relationship between entropy and spontaneity
- Replies: 3
- Views: 1700
Re: Relationship between entropy and spontaneity
Spontaneity refers to any reaction that favors products over reactants. Because so much energy is used to make the products, there's always going to be a decrease in free energy (Gibbs free energy). That is why \Delta G is negative. Entropy must stay positive (or increase) in order to keep \Delta G ...
- Sun Jan 28, 2018 2:35 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Reversible vs Irreversible
- Replies: 2
- Views: 382
Re: Reversible vs Irreversible
Right, reversible expansion focuses more on the matter that all heat is used toward work. As far as I've seen, there hasn't been any experiments looking into the matter.
- Sun Jan 28, 2018 2:27 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: CV or CP
- Replies: 5
- Views: 648
Re: CV or CP
Cv refers to constant volume, whereas Cp refers to constant pressure. If anything, the problem may describe which one you're going to use depending on whether the system is described as isobaric(constant pressure) or Isometric (constant volume).
- Sun Jan 21, 2018 11:59 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: heat capacity
- Replies: 2
- Views: 385
heat capacity
What's the difference between molar and specific heat capacity?
- Sun Jan 21, 2018 11:44 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Heat and systems
- Replies: 3
- Views: 554
Heat and systems
How can you tell whether or not heat is being transferred to the system or from the system?
- Sun Jan 21, 2018 11:40 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: System Types
- Replies: 7
- Views: 873
System Types
Can someone please give me specific examples of each type of system(open, closed, isolated) and why they're that way? It's difficult to grasp the difference between them.
- Thu Jan 11, 2018 1:58 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Enthalpy of sublimation
- Replies: 3
- Views: 789
Enthalpy of sublimation
So in lecture Lavelle explained that the change of enthalpy in sublimation is equal to the sum of enthalpy of fusion and the enthalpy of vapor. Can someone please explain to me why this is so?
- Thu Jan 11, 2018 1:52 pm
- Forum: Phase Changes & Related Calculations
- Topic: Enthalpy of Intramolecular Forces
- Replies: 3
- Views: 869
Re: Enthalpy of Intramolecular Forces
That's a good question! I'm actually curious about that as well. Although Van Der Waal forces are relatively weak and fluctuate due to the movement of electrons, I think energy is still released/absorbed in any phase change.
- Mon Jan 08, 2018 5:47 pm
- Forum: Phase Changes & Related Calculations
- Topic: Endothermic Reaction
- Replies: 10
- Views: 9548
Endothermic Reaction
In lecture today, Lavelle mentioned the melting of an ice cube being an endothermic reaction. I'm just a tad confused as to why this is?
- Sun Dec 10, 2017 12:26 am
- Forum: DeBroglie Equation
- Topic: Measurable Values
- Replies: 1
- Views: 227
Re: Measurable Values
In this case, I'm pretty sure as long as velocity is under the speed of light, it's considered a reasonable value.
- Sun Dec 10, 2017 12:23 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3614848
Re: Post All Chemistry Jokes Here
Sam Smoot 1L wrote:Don't be salty
That was sodium funny! I slapped my neon that one!
- Sun Dec 10, 2017 12:22 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3614848
Re: Post All Chemistry Jokes Here
Let's take all these bad chemistry jokes and Barium.
- Thu Dec 07, 2017 7:35 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: ClF3 Lewis acid/ base?
- Replies: 2
- Views: 2221
Re: ClF3 Lewis acid/ base?
This is correct. Having three Flourine surrounding one Chlorine leaves two electron pairs that's available to be donated. This is because chlorine has a total of 7 valence electrons. So with the three bonding Flourine giving three valence electrons, there has to be 2 more lone pairs in order to give...
- Thu Dec 07, 2017 7:12 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Isoelectrinic
- Replies: 2
- Views: 477
Re: Isoelectrinic
Isoelectronic ions consist of two molecules that have both the same amount of atoms and valence electrons. In Na+ and F-, since Na loses an electron, it goes into group 18, where it has 8 valence electrons and 10 atoms rather than 11. For F-, adding an electron makes F go into group 18 as well. So t...
- Wed Dec 06, 2017 2:23 pm
- Forum: Limiting Reactant Calculations
- Topic: Limiting reagent [ENDORSED]
- Replies: 3
- Views: 494
Limiting reagent [ENDORSED]
I'm still confused as to how to determine which molecule is the limiting reagent. How do I find the ratio in order to determine which molecule would be the limiting reagent?
- Sat Dec 02, 2017 8:31 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Molecular Orbital Theory
- Replies: 4
- Views: 443
Re: Molecular Orbital Theory
Since there wasn't any mention of MO theory during lectures or on the online site, I don't believe there will be any specific questions regarding this.
- Sat Dec 02, 2017 8:26 pm
- Forum: Hybridization
- Topic: Pi Bonds
- Replies: 2
- Views: 305
Pi Bonds
What does it mean when a pi bond is delocalized?
- Sat Oct 28, 2017 5:34 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: d orbital [ENDORSED]
- Replies: 2
- Views: 369
d orbital [ENDORSED]
Why do elements in the d orbital begin with 3d instead of 4d, since the d orbital begins in the 4th period?
- Tue Oct 10, 2017 5:36 pm
- Forum: Administrative Questions and Class Announcements
- Topic: weekly online discussion?
- Replies: 1
- Views: 246
Re: weekly online discussion?
You have the post correct, seeing as you've just posted a question. For each post, you get one point that counts towards your grade. You can only have a maximum of 2 points per week, but you'll come to find that you're posting more than twice on this page. The week ends on Sunday 11:59 pm, and then ...

