Search found 22 matches
- Sun Jul 30, 2017 11:29 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Reaction Quotient
- Replies: 10
- Views: 1012
Re: Reaction Quotient
The reaction quotient (Q) is similar to the equilibrium constant (K), but K is the constant when the reaction is in equilibrium while Q is for any stage of the reaction except when it is at equilibrium.
- Sun Jul 30, 2017 11:26 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Example from Pg 134 in Course Reader
- Replies: 1
- Views: 383
Re: Example from Pg 134 in Course Reader
KP = PPCl3 PCl2 / PPCl5
KP (PPCl5 / PCl2) = PPCl3 (PCl2 / PPCl5)(PPCl5 / PCl2)
KP PPCl5 / PCl2 = PPCl3
KP (PPCl5 / PCl2) = PPCl3 (PCl2 / PPCl5)(PPCl5 / PCl2)
KP PPCl5 / PCl2 = PPCl3
- Sun Jul 30, 2017 7:48 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Change
- Replies: 2
- Views: 443
Re: Change
Because the reactants are being used to make the product, the equilibrium concentration of the reactants would be less than the initial while the product's equilibrium concentration would be higher than its initial. Since we don't know the amount of reactants used to make the product, we would put i...
- Sun Jul 30, 2017 7:42 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Ka and Kb values
- Replies: 3
- Views: 645
Re: Ka and Kb values
They will probably be given.
- Sat Jul 22, 2017 10:52 pm
- Forum: Hybridization
- Topic: Question 4.39 [ENDORSED]
- Replies: 4
- Views: 979
Re: Question 4.39 [ENDORSED]
P4 is nonpolar because the 4 P atoms are arranged about a center of symmetry and they have the same electronegativity.
- Sat Jul 22, 2017 10:43 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Positive Concentrations
- Replies: 1
- Views: 316
Re: Positive Concentrations
Yes, but you should still try plugging in both numbers just to make sure. :)
- Sat Jul 22, 2017 4:18 pm
- Forum: Hybridization
- Topic: HW Problem 4.77
- Replies: 3
- Views: 663
Re: HW Problem 4.77
We skipped molecular orbital theory so I don't think we have to do this problem.
- Sat Jul 22, 2017 4:11 pm
- Forum: Hybridization
- Topic: Homework Problem 4.43
- Replies: 2
- Views: 690
- Sat Jul 22, 2017 1:59 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3649028
- Sat Jul 15, 2017 7:06 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Relationship between Electronegativity and Orbital Energy
- Replies: 4
- Views: 3894
Re: Relationship between Electronegativity and Orbital Energy
Electronegativity is directly proportional to effective nuclear charge, so when electronegativity is high, the nuclear charge is high as well. The high nuclear charge strongly attracts the electrons and pulls them in, decreasing the energy of the orbitals.
- Sat Jul 15, 2017 3:58 pm
- Forum: Lewis Structures
- Topic: Octet Rule [ENDORSED]
- Replies: 9
- Views: 1551
Re: Octet Rule [ENDORSED]
One exception to the octet rule would be when atoms exceed their octet. Starting from phosphorus in period 3, atoms can utilize their d subshell in bonding.
- Sat Jul 15, 2017 3:54 pm
- Forum: Ionic & Covalent Bonds
- Topic: The use of hidden d orbital
- Replies: 2
- Views: 543
Re: The use of hidden d orbital
In molecules like 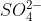 in which sulfur exceeds its octet, the remaining electrons would go into the d orbitals, which should be filled in the same way the s and p orbitals are filled using the Pauli exclusion principle and Hund's rule.
in which sulfur exceeds its octet, the remaining electrons would go into the d orbitals, which should be filled in the same way the s and p orbitals are filled using the Pauli exclusion principle and Hund's rule.
- Sat Jul 15, 2017 2:44 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Naming Compounds
- Replies: 1
- Views: 443
Re: Naming Compounds
I suppose the arrangement containing the lone pairs is called the electron geometry and the actual shape of the molecule is called the molecular geometry. This is a great simulation that shows both the electron and molecular geometry: https://phet.colorado.edu/sims/html/molecule-shapes/latest/molecu...
- Sat Jul 08, 2017 5:24 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Ground state vs. excited state
- Replies: 3
- Views: 69958
Re: Ground state vs. excited state
The ground state electron configuration of an element is the same as the regular configuration in which the electrons are in the lowest possible energy state. For example, the ground state electron configuration of oxygen is 1s2 2s2 2p4. The excited state electron configuration shows when an electro...
- Sat Jul 08, 2017 4:56 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Graphs on page 44 of the textbook
- Replies: 1
- Views: 482
Re: Graphs on page 44 of the textbook
This isn't a complete explanation but here's a list of what I have observed from looking at these graphs. The radial distribution function equals zero when ever the radius is equal to zero, signifying that the probability of finding an electron at the nucleus is zero. It can be seen that radii of th...
- Sat Jul 08, 2017 4:20 pm
- Forum: Trends in The Periodic Table
- Topic: Question 2.81
- Replies: 1
- Views: 770
Re: Question 2.81
The electron configuration of nitrogen is 1s2 2s2 2p3, which shows that each p-orbital contains one electron. However, the electron configuration of oxygen is 1s2 2s2 2p4, which shows that the px orbital contains two electrons. This leads to electron-electron repulsion which causes instability and m...
- Sat Jul 08, 2017 3:46 pm
- Forum: Bond Lengths & Energies
- Topic: ionization energy
- Replies: 2
- Views: 600
Re: ionization energy
Na has a smaller first ionization energy than Al because Al has a greater nuclear charge than Na, which causes the electrons to be more attracted to the center. Also, ionization energy tends to decrease down a group and increase across a period. The first ionization energy is the amount of energy it...
- Sun Jul 02, 2017 7:17 pm
- Forum: DeBroglie Equation
- Topic: Question about 1.33
- Replies: 4
- Views: 877
Re: Question about 1.33
Oh, I thought you were asking about part C. You would use 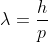 to solve for the wavelength. How did you get E=vh/λ ?
to solve for the wavelength. How did you get E=vh/λ ?
- Sat Jul 01, 2017 10:50 pm
- Forum: DeBroglie Equation
- Topic: Question about 1.33
- Replies: 4
- Views: 877
Re: Question about 1.33
The question doesn't ask for the wavelength of an ejected electron. It asks for the wavelength of the photon that caused the ejection of an electron.
- Sat Jul 01, 2017 3:49 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Problem E.15 in Fundamentals
- Replies: 3
- Views: 697
Re: Problem E.15 in Fundamentals
Find the molar mass of the unknown element M by subtracting the mass of the hydrogen and the oxygen atoms from the given molar mass of the molecule: 74.10 - 2(1.008 + 15.999) = 40.086 g/mol This molar mass is the closest to the molar mass of Calcium, so we can assume that M is calcium. Calcium sulfi...
- Sat Jul 01, 2017 3:35 pm
- Forum: DeBroglie Equation
- Topic: Question about 1.33 Part C
- Replies: 1
- Views: 607
Re: Question about 1.33 Part C
Use E\left ( photon \right )=E\left ( threshold \right )+E_{k} Find the energy of the photon: E\left ( photon \right )=E\left ( threshold \right )+\frac{1}{2}m_{e}v_{e}^{2} E\left ( photon \right )=\left ( 1.66\times 10^{-17} J\right )+\frac{1}{2}\left...
- Sat Jul 01, 2017 1:37 pm
- Forum: Empirical & Molecular Formulas
- Topic: Whole Number Coefficients
- Replies: 5
- Views: 1472
Re: Whole Number Coefficients
You can also convert them to fractions and multiply by the denominator.
For example, you can convert 1.666 to and multiply by 3 to get 5.
and multiply by 3 to get 5.
For example, you can convert 1.666 to


