Search found 25 matches
- Sun Jul 30, 2017 1:37 pm
- Forum: Conjugate Acids & Bases
- Topic: Question 12.3
- Replies: 2
- Views: 662
Re: Question 12.3
Yes, it is required to write the state of matter that the components are in. Usually, the solvent will be written as a liquid and the other substances are aqueous because they are mixed with the water.
- Sun Jul 30, 2017 1:34 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: calculating K
- Replies: 2
- Views: 450
Re: calculating K
I think it just depends on what unit the question is asking for.
- Sun Jul 30, 2017 1:31 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3660274
Re: Post All Chemistry Jokes Here
Q: What is the chemical formula for "banana"?
A: BaNa2
A: BaNa2
- Fri Jul 28, 2017 4:59 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: 11.33
- Replies: 4
- Views: 914
Re: 11.33
You divide the moles by 0.500 because you need to find the concentration of the various gases by using: concentration = moles/volume (liters).
- Fri Jul 28, 2017 10:08 am
- Forum: Naming
- Topic: Naming Ligands
- Replies: 2
- Views: 743
Re: Naming Ligands
You only add "ion" when naming a ligand that is a coordinate covalent compound by itself as an ion without binding to a respective anion or cation.
For example, [Co(NH3)5Cl]+ would be labeled as pentaamminechlorocobaltate(II) ion.
For example, [Co(NH3)5Cl]+ would be labeled as pentaamminechlorocobaltate(II) ion.
- Fri Jul 28, 2017 10:03 am
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Course Reader Example on Pg. 154
- Replies: 2
- Views: 497
Re: Course Reader Example on Pg. 154
As Dr. Lavelle stated, the negative charge of the oxygen is stabilized more with the chlorine atoms in the compound because chlorine is a very electronegative atom.
- Fri Jul 28, 2017 9:58 am
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: homework 12.39
- Replies: 1
- Views: 521
Re: homework 12.39
Two criteria that you can use to determine the relative acidity of acidic compounds is by looking at the length of the bonds and the stability of the resulting anion after the H+ atom is removed.
- Fri Jul 21, 2017 10:22 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3660274
Re: Post All Chemistry Jokes Here
What is the chemical formula for "coffee"?
CoFe2
CoFe2
- Fri Jul 21, 2017 10:17 pm
- Forum: Lewis Structures
- Topic: Lewis Structure
- Replies: 2
- Views: 537
Re: Lewis Structure
The order of the atoms in the molecular compound can also aid in the formation of the Lewis structure. For example, the COOH at the end of the formula illustrates that there will be a carboxylic acid attached to the nitrogen.
- Fri Jul 21, 2017 10:14 pm
- Forum: Lewis Structures
- Topic: double bonds
- Replies: 2
- Views: 501
Re: double bonds
For an atom such as Oxygen and Sulfur, a double bond is sometimes needed in order to satisfy a full bond and to achieve a 0 (most stable) formal charge.
- Fri Jul 21, 2017 10:12 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Exercise 3.13
- Replies: 2
- Views: 648
Re: Exercise 3.13
a) 4s
b) 3p
c) 3p
d) 4s
b) 3p
c) 3p
d) 4s
- Sun Jul 16, 2017 8:10 am
- Forum: Lewis Structures
- Topic: Octet Rule [ENDORSED]
- Replies: 9
- Views: 1551
Re: Octet Rule [ENDORSED]
Another exception would be when when an atom has too little electrons in a compound. BeCl2, for example, is a compound in which the central atom does not possess an octet.
- Sun Jul 16, 2017 8:05 am
- Forum: Lewis Structures
- Topic: Question 3.39
- Replies: 1
- Views: 533
Re: Question 3.39
For the compounds that you listed in the problem, the cation is always written first whereas the anion is written second. When writing the anion, replace the end of the anion with the suffix, -ide. For example, chlorine would be written as chloride. For organic compounds such as CH4 or Methane, ther...
- Sat Jul 15, 2017 8:53 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Problem 2.47
- Replies: 3
- Views: 984
Re: Problem 2.47
Be sure that when removing electrons, the electrons from the ns state are removed first before removing those from the (n-1)d state. (e.g. 4s state electrons before 3d electrons)
- Sat Jul 15, 2017 8:37 am
- Forum: Ionic & Covalent Bonds
- Topic: Polyatomic Lewis Structures
- Replies: 5
- Views: 1444
Re: Polyatomic Lewis Structures
If a molecule has a charge of 3+, it means that the molecule has 3 less electrons than it normally does. This would mean that you use 3 electrons less on the diagram than you would if you were drawing the ground state diagram. On the other hand, if a molecule were to have a 3- charge, then it would ...
- Tue Jul 11, 2017 3:55 pm
- Forum: Photoelectric Effect
- Topic: Photoelectric Effect Module Problem
- Replies: 1
- Views: 504
Re: Photoelectric Effect Module Problem
1. Use 194 nm, convert it to meters, and solve for frequency using c=wavelength x frequency 2. Use E=hv and solve for the energy. This energy is the energy of a photon that is radiated onto the surface 3. Next, use the formula E(kinetic) = E(photon) - E(threshold) to solve for the kinetic energy Sin...
- Tue Jul 11, 2017 3:49 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Text Book Problem 2.29 (d)
- Replies: 3
- Views: 762
Re: Text Book Problem 2.29 (d)
The question is asking about how many electrons can have the quantum number of n=2, meaning the 2nd shell. Since the 2nd shell possesses only s and p subshells, those subshells are the only ones that can have the number n=2. We learned that s- subshells have one orbital and that each orbital can hol...
- Fri Jul 07, 2017 10:33 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Atomic Spectra & Energy Levels
- Replies: 2
- Views: 617
Re: Atomic Spectra & Energy Levels
In a mathematical sense, the limit of the function 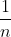 as n approaches infinity is 0 (same principle applies for
as n approaches infinity is 0 (same principle applies for 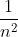 ), so the energy (E) value will go to 0 when n approaches infinity.
), so the energy (E) value will go to 0 when n approaches infinity.
- Fri Jul 07, 2017 10:15 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: True Or False??
- Replies: 2
- Views: 658
Re: True Or False??
Wu is correct. When electrons in an atom move from a higher state to a lower one (when electromagnetic radiation is emitted), they emit energy; and thus, the energy of the atom does not increase but rather decreases. On the other hand, the energy of an atom increases when it absorbs electromagnetic ...
- Fri Jul 07, 2017 10:07 pm
- Forum: Properties of Light
- Topic: Chapter 1, problem 3 [ENDORSED]
- Replies: 10
- Views: 1486
Re: Chapter 1, problem 3 [ENDORSED]
I believe you are correct in that the speed of light is a constant so it should be the same for all photons regardless of the frequency. However, the case would be different with particles that have rest mass as De Broglie's equation includes velocity to solve for the wavelength of a particle, which...
- Wed Jul 05, 2017 11:31 pm
- Forum: Student Social/Study Group
- Topic: Self Test 1 Answers
- Replies: 8
- Views: 1585
Re: Self Test 1 Answers
Ben's answer for number 5 is correct. Dr. Lavelle used C3HO4 as an example in the post when he divided the respective number of moles by the lowest value of moles.
- Thu Jun 29, 2017 11:13 pm
- Forum: Empirical & Molecular Formulas
- Topic: How can we write the molecular formulas of some organic chemicals?
- Replies: 3
- Views: 627
Re: How can we write the molecular formulas of some organic chemicals?
Additionally, the order that the respective elements are given in a molecular formula gives insight on the specific formation that the atoms are placed in.
- Wed Jun 28, 2017 5:19 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Question on Problem E.1 in textbook [ENDORSED]
- Replies: 2
- Views: 546
Re: Question on Problem E.1 in textbook [ENDORSED]
You would need to find the diameter of the atoms instead of the circumference because the question is asking for the length of the stringed atoms, which is found by adding the diameters, and not the circumference of all the atoms.
- Wed Jun 28, 2017 5:16 pm
- Forum: Properties of Light
- Topic: energy required to remove an electron [ENDORSED]
- Replies: 7
- Views: 3922
Re: energy required to remove an electron [ENDORSED]
The energy required to remove an electron is the value of sodium's work function, so the value would be 150.6 kj/mol.
- Wed Jun 28, 2017 5:09 pm
- Forum: Properties of Light
- Topic: When using the constant C
- Replies: 2
- Views: 528
Re: When using the constant C
Dr. Lavelle said that he rounded the value of the speed of light (c) to 3.00x10^8 in his lecture slide, so I would assume that it would be okay to use the value.

