Search found 58 matches
- Fri Mar 16, 2018 11:08 am
- Forum: General Science Questions
- Topic: Class [ENDORSED]
- Replies: 3
- Views: 716
Re: Class [ENDORSED]
No class today.
- Mon Mar 12, 2018 10:22 am
- Forum: *Enzyme Kinetics
- Topic: Enzymes
- Replies: 8
- Views: 1715
Re: Enzymes
We learned about mechanisms earlier. Some enzymes (maybe even all enzymes) lower the energy by creating a different mechanism for the reaction. This new mechanism has a smaller energy barrier.
- Mon Mar 12, 2018 10:20 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: What is A?
- Replies: 4
- Views: 535
Re: What is A?
We don't have a way to calculate A without using the equation we saw in class and on our formula sheet.
- Mon Mar 12, 2018 10:15 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagram Order for Same Phases
- Replies: 4
- Views: 612
Re: Cell Diagram Order for Same Phases
I think Lavelle prefers reactants listed before products, but I don't expect this to be graded too strictly.
- Mon Mar 12, 2018 10:07 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Heat capacity question
- Replies: 5
- Views: 729
Re: Heat capacity question
These are ways to approximate the heat capacity of a gas without needing to be given experimental data.
- Tue Mar 06, 2018 2:23 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Reaction Mechanisms on test 3?
- Replies: 2
- Views: 339
Re: Reaction Mechanisms on test 3?
This will not be on test 3
- Tue Mar 06, 2018 2:20 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Which compound to use
- Replies: 3
- Views: 424
Re: Which compound to use
Lavelle stated in class that it would take a lot of chemistry knowledge to come up with an accurate intermediate on your own. He doesn't expect us to do that.
- Tue Mar 06, 2018 2:18 pm
- Forum: Experimental Details
- Topic: Rate Unit Conversion
- Replies: 4
- Views: 1123
Re: Rate Unit Conversion
The rate has to have units that match with the rate constant, k. k usually has s^-1 in its units, but there is a chance that a problem will give a constant with minutes or something.
- Tue Mar 06, 2018 2:13 pm
- Forum: Experimental Details
- Topic: units
- Replies: 4
- Views: 991
Re: units
Some problems ask for answers in specific units, so it's important to read the question carefully.
- Fri Mar 02, 2018 1:20 pm
- Forum: First Order Reactions
- Topic: Fractional Order Reactions [ENDORSED]
- Replies: 3
- Views: 1226
Re: Fractional Order Reactions [ENDORSED]
Although those are possible, we don't have to worry about them.
- Fri Mar 02, 2018 1:17 pm
- Forum: General Rate Laws
- Topic: Reactions higher than the second order [ENDORSED]
- Replies: 3
- Views: 509
Re: Reactions higher than the second order [ENDORSED]
Will we be talking about negative order, mixed order, or fractional order?
Probably not. We don't have to worry about that, unless he mentions it in class.
- Fri Mar 02, 2018 1:09 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Fractional Reaction Orders [ENDORSED]
- Replies: 2
- Views: 412
Re: Fractional Reaction Orders [ENDORSED]
I do not think that we will deal with non-interger reaction orders in this class.
- Fri Mar 02, 2018 1:07 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Average rate [ENDORSED]
- Replies: 6
- Views: 1026
Re: Average rate [ENDORSED]
We assume that the reaction is going in the forward direction
- Fri Mar 02, 2018 1:01 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Straight line
- Replies: 7
- Views: 1765
Re: Straight line
Each order produces a straight line when the y axis is set to a specific function of concentration.
- Thu Feb 22, 2018 9:00 pm
- Forum: Balancing Redox Reactions
- Topic: Does order matter?
- Replies: 7
- Views: 942
Re: Does order matter?
We haven't learned any convention for that specific order, but I would imagine that a rule likely exists for making very long reactions more organized. We don't have to worry about it for this class, though.
- Thu Feb 22, 2018 8:58 pm
- Forum: Balancing Redox Reactions
- Topic: Acidic and Basic Solution
- Replies: 3
- Views: 505
Re: Acidic and Basic Solution
This should be given in the problem.
- Wed Feb 21, 2018 9:13 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 14.15
- Replies: 1
- Views: 293
14.15
In the answer book, the cell diagram shows:
CD(s) | Cd(OH)2(s) | KOH(aq) || Ni(OH)3(s) | Ni(OH)2(s) | Ni(s)
Why are there bars and not commas in between the solids, since they are the same phase?
CD(s) | Cd(OH)2(s) | KOH(aq) || Ni(OH)3(s) | Ni(OH)2(s) | Ni(s)
Why are there bars and not commas in between the solids, since they are the same phase?
- Tue Feb 20, 2018 2:49 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 14.13b
- Replies: 1
- Views: 169
14.13b
In the cathode of the cell diagram, Ce 4+ (aq) and Ce 3+ (aq) are both the same phase, so they require a conductor, Platinum. But why does the solutions manual put platinum on the anode side as well? I - (aq) and I 2 (s) shouldn't need a conducting metal, right? Is it a rule that we have to add the ...
- Mon Feb 12, 2018 7:54 pm
- Forum: Calculating Work of Expansion
- Topic: Derivations
- Replies: 2
- Views: 394
Re: Derivations
I would recommend knowing the derivations of the Van't Hoff equation and the work equations, just to be safe. He will not ask us to "derive this equation," but he may ask something that would require us to know how the equation is made.
- Mon Feb 12, 2018 7:49 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Gas Constant, R
- Replies: 6
- Views: 829
Re: Gas Constant, R
These values are all equivalent, they are just in different units. Depending on the units of the given information, these other values can be very helpful.
- Mon Feb 12, 2018 7:42 pm
- Forum: Van't Hoff Equation
- Topic: Confusion on different versions of the Van't Hoff equation?
- Replies: 4
- Views: 1636
Re: Confusion on different versions of the Van't Hoff equation?
That is just two ways of writing the same equation. They may look different, but the negative sign is just on different sides of the equation. You can use properties of logs to see why the k2/k1 becomes k1/k2.
- Mon Feb 12, 2018 7:38 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Delta U = 0
- Replies: 2
- Views: 349
Re: Delta U = 0
This is based off the equation 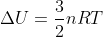 , which shows that with no change in temperature, there would be no resulting change in internal energy.
, which shows that with no change in temperature, there would be no resulting change in internal energy.
- Sat Feb 10, 2018 3:00 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: ∆S total [ENDORSED]
- Replies: 4
- Views: 489
Re: ∆S total [ENDORSED]
∆S tot is the same as the entropy of the universe. According to the Second Law of Thermodynamics, the universe tends to shift towards disorder, so ∆S cannot be less than zero. Thus, a negative ∆S tot is not only nonspontaneous but also considered a forbidden process. This seems to contradict the en...
- Sat Feb 10, 2018 2:15 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: ∆S total [ENDORSED]
- Replies: 4
- Views: 489
Re: ∆S total [ENDORSED]
Yes, but isn't ∆S(total) the entropy of the universe? Because the system + the surroundings = the universe. And the entropy of the universe can never decrease.
- Sat Feb 10, 2018 1:42 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: ∆S total [ENDORSED]
- Replies: 4
- Views: 489
∆S total [ENDORSED]
For a nonspontaneous process, ∆Stotal < 0.
How is it possible for the total entropy to decrease? Since ∆S(total)= ∆S(sys) + ∆S(surr), doesn't that mean that ∆S(total) is the ∆S of the universe?
How is it possible for the total entropy to decrease? Since ∆S(total)= ∆S(sys) + ∆S(surr), doesn't that mean that ∆S(total) is the ∆S of the universe?
- Sat Feb 10, 2018 11:50 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Van't Hoff Equation
- Replies: 3
- Views: 421
Van't Hoff Equation
The equation is not listed on the formula sheet. Does this mean that we need to know how to derive the equation for the midterm?
- Fri Feb 09, 2018 10:15 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy and Equilibrium
- Replies: 1
- Views: 280
Gibbs Free Energy and Equilibrium
I think we learned that at equilibrium, ∆G=0.
But the equation ∆G=-RTln(k) seems to contradict this, since it is calculating a nonzero ∆G value at equilibrium. Are these two ∆G values different?
But the equation ∆G=-RTln(k) seems to contradict this, since it is calculating a nonzero ∆G value at equilibrium. Are these two ∆G values different?
- Fri Feb 09, 2018 10:05 am
- Forum: Biological Examples (*DNA Structural Transitions, etc.)
- Topic: Biological systems
- Replies: 7
- Views: 1509
Re: Biological systems
One reaction releases energy (like breaking ATP). This energy can be used to drive a reaction that is not favorable (like pumping ions across a barrier).
- Fri Feb 09, 2018 10:02 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: oxygen has 0 Gibbs free energy of formation
- Replies: 2
- Views: 5514
Re: oxygen has 0 Gibbs free energy of formation
Unlike energy, entropy can never be zero (unless you are at 0K).
- Wed Feb 07, 2018 11:02 am
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Residual Entropy
- Replies: 2
- Views: 359
Re: Residual Entropy
At 0K, there is no movement. The only disorder is resulting from position of the molecules. That is why residual entropy is positional entropy.
- Fri Feb 02, 2018 6:09 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Lavelle Office Hours
- Replies: 1
- Views: 236
Lavelle Office Hours
We were sent an email saying "office hours are Wednesday's 2-4pm."
What about the Friday office hours? I am unsure if this is stating that office hours will only be on Wednesdays from now on or if this means that Fridays are staying the same and Wednesdays are changing slightly.
What about the Friday office hours? I am unsure if this is stating that office hours will only be on Wednesdays from now on or if this means that Fridays are staying the same and Wednesdays are changing slightly.
- Mon Jan 29, 2018 9:20 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 9.13
- Replies: 3
- Views: 403
Re: 9.13
I thought it was odd that you are expected to assume one mole. It seems like something that you can't assume; you need to be given it? But it this a safe assumption for future problems?
- Mon Jan 29, 2018 9:13 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Kb=?
- Replies: 2
- Views: 339
Re: Kb=?
Boltzman's constant will also be provided on the formula sheet as 1.38x10^23 J/K
- Mon Jan 29, 2018 9:07 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: entropy of heavy vs. light molecules
- Replies: 2
- Views: 858
Re: entropy of heavy vs. light molecules
Molecules with more "parts," such as electrons, protons, and other atoms bonded, have more possible orientations and vibrations than simpler molecules. This means they have more entropy.
- Mon Jan 29, 2018 9:03 pm
- Forum: Administrative Questions and Class Announcements
- Topic: homework/reading schedule
- Replies: 3
- Views: 545
Re: homework/reading schedule
This depends on your TA, but I think we should be doing chapter nine problems this week, since all of our lectures this week will be chapter 9.
- Fri Jan 26, 2018 1:02 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: deltaH Units
- Replies: 5
- Views: 564
Re: deltaH Units
I was under the impression that when calculating the standard reaction enthalpy, we use kJ/mol. For just regular reaction enthalpy, I would put kJ. But I would like to know if that is true or not.
- Wed Jan 24, 2018 6:06 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Combustion
- Replies: 3
- Views: 491
Combustion
When doing combustion problems (such as finding the enthalpy of combustion), is the water produced in the liquid or gaseous state?
Problems usually don't give this information.
Problems usually don't give this information.
- Wed Jan 24, 2018 12:05 pm
- Forum: Calculating Work of Expansion
- Topic: 8.55
- Replies: 2
- Views: 301
Re: 8.55
I encountered the same issue. It is a mistake in the textbook.
- Tue Jan 23, 2018 8:46 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Isothermal expansion
- Replies: 3
- Views: 366
Re: Isothermal expansion
Does ΔU =0 in isothermal expansion?
Isothermal means ΔT = 0, so q=0.
But since there is expansion, doesn't that mean there must be work being done? Doesn't a change in volume guarantee that work is nonzero? I would think that ΔU = w in this situation.
Isothermal means ΔT = 0, so q=0.
But since there is expansion, doesn't that mean there must be work being done? Doesn't a change in volume guarantee that work is nonzero? I would think that ΔU = w in this situation.
- Tue Jan 23, 2018 8:39 pm
- Forum: Phase Changes & Related Calculations
- Topic: How to differentiate when to use certain formulas
- Replies: 3
- Views: 532
Re: How to differentiate when to use certain formulas
Here are the big keywords/hints for the q and w equations.
"Final Temperature" usually suggests using q=mCΔT, since ΔT is Tfinal - Tinitial.
Similarly, when a final and initial volume is given or mentioned, you are likely to use w = PΔV
"Final Temperature" usually suggests using q=mCΔT, since ΔT is Tfinal - Tinitial.
Similarly, when a final and initial volume is given or mentioned, you are likely to use w = PΔV
- Tue Jan 23, 2018 8:33 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.73 and Units for Reaction Enthalpy
- Replies: 1
- Views: 201
8.73 and Units for Reaction Enthalpy
8.73 asks us to calculate the reaction enthalpy using bond enthalpies. In the answer manual, all of the answers are in units of kJ/mol. Normally, reaction enthalpies are just in kJ. Why are they in kJ/mol? Although bond enthalpies are in units of kJ/mol, we are multiplying them by the moles of bonds...
- Tue Jan 23, 2018 3:43 pm
- Forum: Phase Changes & Related Calculations
- Topic: HW 8.1
- Replies: 2
- Views: 278
Re: HW 8.1
We are considering it a closed and fully insulated thermos. Realistically, some heat will be lost, because nobody has invented a perfect insulation. But for the purpose of this problem, we are considering it 100% thermally insulated.
- Mon Jan 22, 2018 9:07 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.93 Part C
- Replies: 1
- Views: 204
Re: 8.93 Part C
I had the same issue. I really don't think it should be positive like the answer book says.
- Sun Jan 21, 2018 2:30 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: ∆nRT in Reactions with no ∆n
- Replies: 2
- Views: 533
∆nRT in Reactions with no ∆n
There are equations where the moles of product is the same as the moles of reactant, so Delta n = 0. If the moles of gas is equal on both sides, does that mean that there is no work being done, since 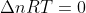 ?
?
- Fri Jan 19, 2018 9:28 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Heat Capcity for Water
- Replies: 8
- Views: 704
Re: Heat Capcity for Water
There are different heat capacities for different states, so you need to know the waters's state. Also, you have molar heat capacity as well as specific heat capacity, and the one you choose depends on the units of your given information.
- Fri Jan 19, 2018 9:26 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 8.29
- Replies: 4
- Views: 276
Re: 8.29
Yes, heat capacity increases with molecular complexity.
- Thu Jan 18, 2018 12:50 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Equations for gases using R
- Replies: 4
- Views: 329
Re: Equations for gases using R
I see PV = nRT as fair game, because it was in Chem14A. However, he has not mentioned anything about this yet in 14B.
- Tue Jan 16, 2018 8:45 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Possible Error in Question 8.55 (Hess' Law)
- Replies: 4
- Views: 500
Re: Possible Error in Question 8.55 (Hess' Law)
[quote="Marina Georgies 1C" Sidenote- where is Dr. Lavelle's list of errors posted? I can't seem to remember.[/quote] It is a PDF on the 14B page titled "Solution Manual Errors" Here it is: https://lavelle.chem.ucla.edu/wp-content/supporting-files/Chem14B/Solution_Manual_Errors_6...
- Tue Jan 16, 2018 8:42 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: standard state of substances
- Replies: 4
- Views: 359
Re: standard state of substances
Most of the diatomic elements are gasses at their standard states; however I2 is a solid, and Br2 is a liquid.
- Tue Jan 16, 2018 8:11 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Acids and Bases
- Replies: 3
- Views: 307
Acids and Bases
The Acids and Bases topic was cut off in my 6-week summer session for Chemistry 14A. Is that topic necessary for 14B? I still plan on learning it eventually either way, as I will probably need it in the future.
- Sat Jan 13, 2018 6:36 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Possible Error in Question 8.55 (Hess' Law)
- Replies: 4
- Views: 500
Possible Error in Question 8.55 (Hess' Law)
In the solution manual for question 55, one of the steps seems to break the rules. In order to get the O 2 molecules to cancel out, the solution manual multiplies one of them by 3/2. However, this coefficient is not multiplied to the rest of the equation; only the oxygen molecule was changed. I do n...
- Fri Jan 12, 2018 12:11 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Answer book
- Replies: 2
- Views: 341
Re: Answer book
The solutions manual and the textbook are the same as in 14A.
- Fri Jan 12, 2018 10:01 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard Enthalpies of Formation of Diatomic Molecules
- Replies: 6
- Views: 4889
Re: Standard Enthalpies of Formation of Diatomic Molecules
So, this rule would only count for the naturally diatomic elements: H, O, F, Cl, Br, I, N?
- Fri Jan 12, 2018 9:09 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard Enthalpies of Formation of Diatomic Molecules
- Replies: 6
- Views: 4889
Standard Enthalpies of Formation of Diatomic Molecules
Why is the standard enthalpy of formation of O2 gas 0kJ/mol?
(This is from example 8.11 on page 296).
(This is from example 8.11 on page 296).
- Wed Jan 10, 2018 8:57 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Notation
- Replies: 4
- Views: 274
Re: Notation
Scientific notation is often used to remove any ambiguity about the number of sig-figs.
- Wed Jan 10, 2018 8:41 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Hess's Law
- Replies: 8
- Views: 1423
Re: Hess's Law
You should be able to change the equations so that you can cancel things. For example, you could reverse the direction of one equation. Or you could multiply an equation's coefficients by 2. These changes would make it possible to cancel something that needs to be cancelled. I do not think there wil...
- Wed Jan 10, 2018 2:36 pm
- Forum: Phase Changes & Related Calculations
- Topic: Temperature during Phase Change
- Replies: 6
- Views: 441
Re: Temperature during Phase Change
The textbook says "At the melting point, the temperature remains constant, because all of the heat is being used to melt the sample."
So there is an energy barrier that must be overcome before a liquid could become a vapor.
So there is an energy barrier that must be overcome before a liquid could become a vapor.
- Wed Jan 10, 2018 2:27 pm
- Forum: Administrative Questions and Class Announcements
- Topic: reading textbook
- Replies: 5
- Views: 676
Re: reading textbook
I am guessing that we are starting at page 278, at the section titled "Enthalpy." That would correspond to questions starting at 8.29.

