Search found 31 matches
- Tue Mar 13, 2018 2:34 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: question 15.47
- Replies: 4
- Views: 467
Re: question 15.47
The solution manual classifies the Cl ion as an intermediate.
- Tue Mar 13, 2018 2:17 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Test 2 #6b
- Replies: 2
- Views: 367
Re: Test 2 #6b
The reducing agent is the species that loses an electron. Ag goes from a neutral state to an oxidation state of +1. AgF stays neutral so this would mean Ag has to be the reducing agent.
- Tue Mar 13, 2018 2:09 pm
- Forum: Balancing Redox Reactions
- Topic: Test 2 #1
- Replies: 6
- Views: 865
Re: Test 2 #1
O has a charge of -2, and since there is 2 molecules of O the total charge of the O2 species is -4. The molecule CO2 is neutral, so that would mean that C has to have a charge of +4 to balance the charge of the O.
- Sat Mar 10, 2018 4:37 pm
- Forum: Zero Order Reactions
- Topic: Zero Order and Catalysts [ENDORSED]
- Replies: 2
- Views: 2869
Re: Zero Order and Catalysts [ENDORSED]
Zero order reactions typically take place when a catalyst is needed for the reaction to occur. The catalyst will be saturated by the reactants and the rate of the reaction will not depend on the concentration of the reactants.
- Sat Mar 10, 2018 3:26 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: SN2 Organic Reaction
- Replies: 3
- Views: 415
Re: SN2 Organic Reaction
Yes. The nucleophile will donate an electron pair to form a chemical bond.
- Sat Mar 10, 2018 3:23 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Molecularity and Coefficients
- Replies: 3
- Views: 1001
Re: Molecularity and Coefficients
The reaction 2 A --> B + C would be bimolecular.
- Wed Feb 28, 2018 7:25 pm
- Forum: First Order Reactions
- Topic: Terminology "first-order" [ENDORSED]
- Replies: 5
- Views: 673
Re: Terminology "first-order" [ENDORSED]
You can refer to the order of individual reactants as well as the order of the reaction. The overall order of the reaction is the sum of the orders of each reactant. So, if each reactant has order 1 then the order of the reaction is 2.
- Wed Feb 28, 2018 5:54 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: units of K
- Replies: 5
- Views: 687
Re: units of K
For 0 order reactions, the units for k are are mol/(L*s). For 1st order reactions, the units of k are s^(-1). For 2nd order reactions, the units for k are L/(mol*s).
- Wed Feb 28, 2018 5:47 pm
- Forum: General Rate Laws
- Topic: Negative Signs [ENDORSED]
- Replies: 6
- Views: 1032
Re: Negative Signs [ENDORSED]
You always want to work with positive reaction rates.
- Sat Feb 24, 2018 11:15 pm
- Forum: General Rate Laws
- Topic: Reactants Rate Law
- Replies: 2
- Views: 368
Re: Reactants Rate Law
In class we discussed that for a recation with one reactant and two products such as aA-->bB+cC that rate= (1/a)(dA/dt) = (1/b)(dB/dt) = (1/c)(dC/dt). So, if there was instead two reactants such as aA+bB--->cC+dD then just account for the additional reactant so, rate= (1/a)(dA/dt) = (1/b)(dB/dt) = (...
- Sat Feb 24, 2018 11:09 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation state
- Replies: 4
- Views: 567
Re: Oxidation state
Yes, you should take into account the coefficients when determining oxidation states.
- Sat Feb 24, 2018 11:07 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 14.41b. Homework b
- Replies: 2
- Views: 402
Re: 14.41b. Homework b
When you get the balanced equation, everything has a stoichiometric coefficient of 2. You can simplify this by dividing the whole balanced equation by 2 which also divides n by 2 resulting in n=1.
- Tue Feb 13, 2018 11:19 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 11.15
- Replies: 2
- Views: 374
Re: 11.15
The question states that the reaction is happening at 1200K so you would use this value to calculate deltaG. The solutions manual calculates delta G not  . This is an important distinction because
. This is an important distinction because  would be calculated at 298K by definition.
would be calculated at 298K by definition.
- Tue Feb 13, 2018 11:14 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 8.33
- Replies: 1
- Views: 338
Re: 8.33
The question asks for Cv. The Cv vaule for polyatomic gases is 3*R. The value 7/2*R is the Cp value for diatomic molecules. You can reference the table on page 281 in the book which lists the Cv and Cp values for ideal gases.
- Tue Feb 13, 2018 11:06 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: practice midterms
- Replies: 2
- Views: 391
Re: practice midterms
There was a practice midterm from a review session posted on chemistry community. You should be able to find it if you search "practice midterm winter 2018".
- Sat Feb 10, 2018 7:47 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Oxidation
- Replies: 4
- Views: 397
Re: Oxidation
Oxidation takes place in the anode(left side) of the galvanic cell.
- Sat Feb 10, 2018 7:45 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Commas in Cell Diagram
- Replies: 2
- Views: 999
Re: Commas in Cell Diagram
A comma is used when the phases of the two elements in contact are the same. For example, you would use a comma for something like Fe3+(aq),Fe2+(aq). A | is used when the phases of the two elements in contact are in different phases. For example: Cu(s)|Cu2+(aq). A | can also indicate that a pourous ...
- Sat Feb 10, 2018 7:39 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: 9.35
- Replies: 2
- Views: 340
Re: 9.35
The problem states that in box A, there is a monoatomic gas, meaning that there are single atoms of gas. The Cv value for atoms is 3/2R. In box B, there are diatomic molecules that are not vibrationally active, meaning these are linear molecules. For linear molecules, the Cv value is 5/2R. In box C,...
- Sat Feb 03, 2018 5:50 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Entropy Changes Due to Change in Pressure
- Replies: 2
- Views: 302
Re: Entropy Changes Due to Change in Pressure
It is always good to remember that pressure is inversely proportional to volume.
- Sat Feb 03, 2018 5:45 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 9.65
- Replies: 6
- Views: 767
Re: 9.65
Write a balanced chemical equation for each compound and calculate the standard entropies of formation. If the standard entropy of formation is negative, then the compound will be less stable at a higher temperature. If the standard entropy of formation is positive, the compound will be more stable ...
- Sat Feb 03, 2018 4:17 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Difference between deltaS(tot), deltaS and deltaS(surr)
- Replies: 5
- Views: 1743
Re: Difference between deltaS(tot), deltaS and deltaS(surr)
I agree with the comments above. It is also worth noting that conceptually, deltaS(tot) is the change in entropy of the universe, the combination of deltaS and deltaS of the surroundings. DeltaS is the change in entropy of the system and deltaS(surr) is the change in entropy of the surroundings, mea...
- Tue Jan 23, 2018 2:53 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard state
- Replies: 2
- Views: 298
Re: Standard state
The standard state of an element is the state it is in at 25 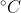 and 1 atm of pressure.
and 1 atm of pressure.
- Tue Jan 23, 2018 2:50 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Heat Capacities of Gas
- Replies: 1
- Views: 217
Re: Heat Capacities of Gas
The equations on page 281 are showing the specific heat capacity for ideal gases under constant volume and constant pressure. When under constant volume, depending on if you're considering an atom, linear molecule, or a non linear molecule, you would use the respective C_{V} value. Similarly, for co...
- Tue Jan 23, 2018 2:39 pm
- Forum: Calculating Work of Expansion
- Topic: work of expansion formula
- Replies: 1
- Views: 293
Re: work of expansion formula
When a system does work of expansion, it is is working against an external pressure and therefore losing energy. Since, \Delta U= q+w , the negative sign ensures that it is understood that the work being done by a system results in a loss of energy since the system is doing work instead of work bein...
- Tue Jan 16, 2018 9:33 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Enthalpy of formation vs reaction enthalpy
- Replies: 2
- Views: 211
Re: Enthalpy of formation vs reaction enthalpy
Reaction enthalpy is the change in heat that occurs when a reaction takes place. To calculate this, you would add the different \Delta H 's of each reaction that took place in order to form the product, i.e. Hess's Law. The enthalpy of formation is the change in enthalpy when an element in its stand...
- Tue Jan 16, 2018 9:21 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.67 part a
- Replies: 1
- Views: 107
Re: 8.67 part a
If you reference table 8.4 in the textbook it lists some standard enthalpies of formation, including water. For water, \Delta H_{f}^{^{\circ}} = -242 kJ/mol. However if you didn't recognize that number, you could also think about how both of the reactants are in a gaseous phase and by adding up the ...
- Tue Jan 16, 2018 9:03 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Heat Capacity: U vs H
- Replies: 2
- Views: 163
Re: Heat Capacity: U vs H
By definition, enthalpy measures the change in heat at constant pressure. So, when finding  , it will always be at constant pressure.
, it will always be at constant pressure.
- Thu Jan 11, 2018 2:58 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Homework Problem 8.21
- Replies: 4
- Views: 375
Re: Homework Problem 8.21
All heat lost by the copper is going to be gained by the water. Therefore, q_{metal}=-q_{water} . q=mC\Delta T , where m=mass, C=specific heat capacity, and \Delta T = the change in temperature.The specifc heat capacity of copper is 0.38 J.^{\circ}C^{-1}.g^{-1} and the specific heat capacity of wate...
- Wed Jan 10, 2018 10:21 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.73a
- Replies: 1
- Views: 230
Re: 8.73a
In this reaction, the C-H bonds are already formed in the C2H2 moleules. Each C2H2 molecule will look like H-C-C-H with the C-C being a triple bond. When the reaction happens, the triple bonds between the carbons are broken and the only new bonds formed are going to be between the carbons since the ...
- Wed Jan 10, 2018 10:07 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Conceptualizing enthalpy
- Replies: 2
- Views: 328
Re: Conceptualizing enthalpy
1. q_{p} is the heat of the system at constant pressure. 2. It might help if you look at it this way: \Delta H = \Delta U +PV . If you assume that the system can only do expansion work then you can substitute in that \Delta U=q+w and w=-P_{ex}\Delta V . Also, because the system is at a constant pres...
- Thu Oct 05, 2017 5:50 pm
- Forum: Balancing Chemical Reactions
- Topic: Fundamentals H.21
- Replies: 2
- Views: 1407
Re: Fundamentals H.21
This is the way I balanced this equation: C10H15N + O2 ---> CO2 + H2O + CH4N2O Step 1 : Balance N since it appears the least 2C10H15N + O2 ---> CO2 + H2O + CH4N2O Step 2: Balance H, there are 30 H on the right side and only 2 H and 4 H, leaving the 4 H alone, that would be 30-4= 26/2= 13 as your coe...

