Search found 51 matches
- Mon Mar 19, 2018 2:02 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Nernst Equation
- Replies: 1
- Views: 601
Re: Nernst Equation
n is the coefficient for the number of electrons in the balanced equation!
- Mon Mar 19, 2018 2:01 am
- Forum: Calculating Work of Expansion
- Topic: reversible and irreversible expansion
- Replies: 3
- Views: 867
Re: reversible and irreversible expansion
If external pressure is constant then you can use the irreversible equation. In addition, the reversible equation is theoretical, so in most "real world" situations, I feel like it's more likely that we would have to use the irreverisble equation.
- Mon Mar 19, 2018 2:00 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: heat capacity
- Replies: 1
- Views: 539
Re: heat capacity
I don't think so... at least we wouldn't have to know them??
- Mon Mar 12, 2018 12:14 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Activation Energy
- Replies: 4
- Views: 598
Re: Activation Energy
I wouldn't necessarily describe this situation as having an "optimum" activation energy. Think of it as, if there is enough energy supplied, then the reactants can reach the activation energy which means they have enough energy to break bonds so that the reaction can occur. If activation e...
- Mon Mar 12, 2018 12:04 am
- Forum: General Rate Laws
- Topic: Rate law constant
- Replies: 4
- Views: 544
Re: Rate law constant
No, it cannot be negative.
- Mon Mar 12, 2018 12:03 am
- Forum: Administrative Questions and Class Announcements
- Topic: Chapters in Final Exam
- Replies: 3
- Views: 740
Re: Chapters in Final Exam
It will cover all of those chapters and I think Dr. Lavelle was saying that the amount of material from a certain chapter that is going to be on the test depends on how much time we spent on it in class. For example, since we breezed through electrochem pretty quickly, it probably won't be the subje...
- Sun Mar 04, 2018 11:47 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Rate determining reaction
- Replies: 3
- Views: 431
Re: Rate determining reaction
^ I agree with them, but if what you're asking is if we need to know that for this week's test, I believe the answer is no. We do not need to know anything past 15.6 (I think that means we don't need to know what Dr. Lavelle taught on Friday)
- Sun Mar 04, 2018 11:45 pm
- Forum: First Order Reactions
- Topic: Derivations
- Replies: 6
- Views: 809
Re: Derivations
I feel like since the tests are usually more straightforward than midterms and finals, we probably won't have to derive an equation. However, Dr. Lavelle did spend a lot of time going over the derivations in class so I guess it wouldn't hurt knowing how to do them just in case!
- Sun Mar 04, 2018 11:43 pm
- Forum: Zero Order Reactions
- Topic: Identifying zero order
- Replies: 6
- Views: 950
Re: Identifying zero order
Yes, when looking at a table you would first make sure the concentration of the reactant you are looking for is the only one changing between two experiments. If the concentration of the reactant changes and the rate does not change, then you know it is 0 order because the concentration of that part...
- Sun Feb 25, 2018 4:53 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Using K
- Replies: 3
- Views: 508
Re: Using K
I think you are getting K confused with k. K is the equilibrium constant. k, what we are using in this section, is the rate constant of just the forward reaction.
- Sun Feb 25, 2018 4:51 pm
- Forum: General Rate Laws
- Topic: Negative sign in reactants
- Replies: 6
- Views: 4732
Re: Negative sign in reactants
Since we are calculating the rate of the forward reaction, we are assuming that the reactants are being used up and converted into the products. Therefore, the concentration of reactants is decreasing and the change in concentration will be negative.
- Sun Feb 25, 2018 4:48 pm
- Forum: General Rate Laws
- Topic: Reaction Rate and Spontaneity
- Replies: 5
- Views: 1508
Re: Reaction Rate and Spontaneity
can a nonspontaneous and spontaneous reaction have the same reaction rate? Possibly? Like if something was added to make the nonspontaneous reaction occur, I don't see why the rate couldn't be the same as that of a spontaneous reaction. I'm not sure if this is correct but that's how I would think o...
- Wed Feb 14, 2018 2:40 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Celsius to Kelvin Clarification
- Replies: 3
- Views: 632
Re: Celsius to Kelvin Clarification
I would use 273.15 on the midterm just to be safe... There's nothing wrong with using a more precise number in your calculations!
- Wed Feb 14, 2018 2:36 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Delta S(tot)
- Replies: 5
- Views: 1289
Re: Delta S(tot)
Aya Shokair- Dis 2H wrote:Varsha Sivaganesh 1A wrote:Also, when the reaction is irreversible,Ssurroundings = 0.
I don't think that's true.
Oh, I have that in my notes from a TA-led review session... maybe I misunderstood what he was saying then.
- Wed Feb 14, 2018 2:06 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Delta S(tot)
- Replies: 5
- Views: 1289
Re: Delta S(tot)
Also, when the reaction is irreversible,  Ssurroundings = 0.
Ssurroundings = 0.
- Sun Feb 11, 2018 1:14 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Higher Molar Entropy?
- Replies: 2
- Views: 393
Re: Higher Molar Entropy?
Bar does correspond to pressure. But I think it's because since there is less pressure, we may be able to assume it is because the volume is greater.
- Sun Feb 11, 2018 1:11 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: 9.13
- Replies: 2
- Views: 319
Re: 9.13
The question says to "assume ideal behavior," so I think that means you assume there is 1 mol of gas!
- Sun Feb 11, 2018 11:14 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Non-spontaneous
- Replies: 4
- Views: 675
Re: Non-spontaneous
If a reaction is spontaneous (\Delta G is negative), that means that the forward reaction is favored. Just remember that this does not mean the reaction will happen quickly, it just means that if you leave the reaction alone, it will complete by itself. So if the forward reaction is non-spontaneous ...
- Sat Feb 03, 2018 9:48 pm
- Forum: Calculating Work of Expansion
- Topic: Calculating Work of Expansion
- Replies: 4
- Views: 680
Re: Calculating Work of Expansion
I would try to remember how to derive the equations Dr. Lavelle shows us, because not only is it possible that it'll be on the test, but it really helps you with understanding the concept and remembering how all of the things we are learning about are related to each other.
- Sat Feb 03, 2018 9:44 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Example from Wednesday's Lecture
- Replies: 3
- Views: 364
Re: Example from Wednesday's Lecture
The reason why we set \Delta G° = 0 is to find out the temperature that the Br2(l) is in equilibrium with the Br2(g). The answer we got for T was 333K. So yes, at 333K, the temperature is at equilibrium and neither the forward nor reverse equation is favored. However, when T > 333K, the forward proc...
- Sat Feb 03, 2018 9:37 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibb's Free Energy
- Replies: 2
- Views: 304
Re: Gibb's Free Energy
\Delta G° is the change in Gibbs free energy at the standard state, meaning at either 1 atm or 1 M. Also, I think that when \Delta G° = 0, the reaction is still at equilibrium... In the example we did in lecture with the Br2(l) turning into Br2(g), we assumed that equilibrium was when \Delta G° = 0...
- Sat Jan 27, 2018 1:38 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Boltzmann's Equation in relation to temperature
- Replies: 4
- Views: 485
Re: Boltzmann's Equation in relation to temperature
I think Boltzmann's equation ignores thermal entropy altogether.
- Sat Jan 27, 2018 1:33 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Spontaneous [ENDORSED]
- Replies: 6
- Views: 724
Re: Spontaneous [ENDORSED]
Well in class, Lavelle mentioned that in any spontaneous process, there is an increase in entropy. However, I'm not sure if that means ALL spontaneous processes have a positive 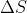
- Sat Jan 27, 2018 1:30 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Ch. 9 Problems, 5
- Replies: 3
- Views: 335
Re: Ch. 9 Problems, 5
For these questions, does anyone know if we have to convert kJ to J to calculate entropy so that its units are J/K? Or can we leave it as kJ/K?
- Sat Jan 27, 2018 1:26 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: reversible and irreversible reactions
- Replies: 4
- Views: 607
Re: reversible and irreversible reactions
Usually, if it is reversible and isothermal then the problem will probably indicate that the temperature is constant. In an irreversible expansion, the external pressure is constant.
- Sun Jan 21, 2018 2:57 pm
- Forum: Phase Changes & Related Calculations
- Topic: Equations to know
- Replies: 6
- Views: 459
Re: Equations to know
K Stefanescu 2I wrote:Will we also be given all of the enthalpy values needed to solve a problem? Are there ANY enthalpy values we should memorize (besides which ones are zero)?
I don't think there's any we have to memorize!
- Sun Jan 21, 2018 2:56 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: How to calculate bond enthalpy
- Replies: 1
- Views: 1085
How to calculate bond enthalpy
How do you calculate bond enthalpies (for example, like in 8.67)? I thought it was products - reactants but for part a it looks like it's reactants - products?
- Sun Jan 21, 2018 2:47 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Equipartition theorem and Degrees of Freedom
- Replies: 1
- Views: 219
Re: Equipartition theorem and Degrees of Freedom
Is it on the homework? I also don't remember him going over that in class, so I'm gonna guess that we probably don't need to know it!
- Fri Jan 12, 2018 9:48 pm
- Forum: Phase Changes & Related Calculations
- Topic: Methods of Enthalpy
- Replies: 8
- Views: 879
Re: Methods of Enthalpy
Dylan Mai 1D wrote:There are 3 methods we know of right now correct?
Yes! The first method is using Hess's method, the second is using bond enthalpies, and the third is using standard enthalpies of formation. So it just depends on what information the question gives you.
- Fri Jan 12, 2018 6:03 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Are there any periodic trends for enthalpy?
- Replies: 4
- Views: 346
Re: Are there any periodic trends for enthalpy?
Even if there are, I don't think you need to worry about them :)
- Fri Jan 12, 2018 5:59 pm
- Forum: Phase Changes & Related Calculations
- Topic: Heating Curve
- Replies: 3
- Views: 379
Re: Heating Curve
There is not a change in temperature because the heat that is added at the plateaus is being used to break apart bonds in order to turn the solid into a liquid, or a liquid into a vapor.
- Fri Dec 08, 2017 5:04 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Calculating the initial concentration without x
- Replies: 1
- Views: 302
Re: Calculating the initial concentration without x
It was because we were given the equilibrium concentration of the final product and the initial concentrations of the reactants. When doing an ice table, if you aren't given the initial concentration of the product, you assume it is 0. So for the problem that Dr. Lavelle did in class, the initial co...
- Wed Dec 06, 2017 5:40 am
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Pka and Pkb values
- Replies: 2
- Views: 501
Re: Pka and Pkb values
I think that we will be given the information necessary to answer the question. For example, if we have to calculate the pKa, then we will either be given the Ka or a way to calculate it. But I'm pretty sure there is no need to memorize pKa/pKb/Ka/Kb or any constants like those!
- Wed Dec 06, 2017 5:37 am
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Percent ionization
- Replies: 3
- Views: 543
Percent ionization
Hello,
is percent deprotonation the same thing as percent ionization? One of the TAs referred to it as percent deprotonation and I've never heard of that before so I was just wondering if they are the same :) If not, what's the difference?
is percent deprotonation the same thing as percent ionization? One of the TAs referred to it as percent deprotonation and I've never heard of that before so I was just wondering if they are the same :) If not, what's the difference?
- Mon Nov 27, 2017 3:11 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Seesaw
- Replies: 2
- Views: 466
Re: Seesaw
The different bond angles are 120 degrees and 90 degrees. I believe he 90 degree angles are between the axial bond and the lone pair electrons.
- Mon Nov 27, 2017 3:08 pm
- Forum: Hybridization
- Topic: Numbers in front of hybrid orbitals [ENDORSED]
- Replies: 2
- Views: 537
Re: Numbers in front of hybrid orbitals [ENDORSED]
Would you just get the number in front from what row the atom is in the periodic table? For example, for 2sp3, the element is in the second row?
- Mon Nov 27, 2017 3:06 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Solids when using ICE [ENDORSED]
- Replies: 8
- Views: 3389
Re: Solids when using ICE [ENDORSED]
So the only things that you would use for the ICE table are aqueous and gases?
- Mon Nov 27, 2017 3:01 pm
- Forum: Naming
- Topic: Ligand order in coordination sphere
- Replies: 3
- Views: 447
Re: Ligand order in coordination sphere
I think in lecture, Dr. Lavelle said that you would put the ligand names in alphabetical order (ex: ammine) but when writing the formulas, I don't think there is a specific order that is needed (ex: NH3)
- Thu Nov 09, 2017 8:59 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: More Polarizable/Polarizing Power
- Replies: 6
- Views: 837
Re: More Polarizable/Polarizing Power
You could also think of it as smaller cations having higher polarizing power!
- Thu Nov 09, 2017 8:57 pm
- Forum: Trends in The Periodic Table
- Topic: Radius
- Replies: 6
- Views: 798
Re: Radius
However, when comparing radii, you would use the same trend whether its atomic, ionic, or covalent radii.
- Fri Nov 03, 2017 5:14 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 3D shape Lewis Diagrams
- Replies: 4
- Views: 885
Re: 3D shape Lewis Diagrams
No, you can draw that if you would like to, but a regular Lewis dot structure will suffice. :) As long as you can also identify the shape and bond angles, I think you're good
- Fri Nov 03, 2017 4:53 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Only certain central atoms have a full octet?
- Replies: 4
- Views: 745
Re: Only certain central atoms have a full octet?
Usually it has to do with the formal charge. Some atoms work better when they don't have an octet because it makes their formal charge closer to 0. For example, if boron is bonded to three fluorine atoms via single bonds, the formal charge of all of those atoms is 0. However, if you were to give bor...
- Wed Oct 25, 2017 3:28 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Possible Quantum numbers given n=6
- Replies: 3
- Views: 766
Re: Possible Quantum numbers given n=6
Unless they tell you whether it is an s, p, d, or f orbital, you would just say that there are 5 possible l values and l could be 0, 1, 2, 3, 4, or 5. But unless they give you the letter I don't think there is any way of knowing what l's value is.
- Wed Oct 25, 2017 3:21 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Configuration writing
- Replies: 8
- Views: 881
Re: Electron Configuration writing
For the test we will only need to know how to do the electron configurations for period 1-4, so I wouldn't worry about Rhodium! And I believe Copper and Chromium are the only exceptions we need to know about, just keep in mind that they are not the only two exceptions in the entire periodic table :)
- Fri Oct 20, 2017 10:01 am
- Forum: *Shrodinger Equation
- Topic: HΨ=EΨ [ENDORSED]
- Replies: 5
- Views: 4507
Re: HΨ=EΨ [ENDORSED]
So we just need to know that Schrodinger's equation is used to find n, l, and ml?? Or is that not what it's used for...?
- Fri Oct 20, 2017 9:57 am
- Forum: DeBroglie Equation
- Topic: When to use De Broglie Equation [ENDORSED]
- Replies: 11
- Views: 5234
Re: When to use De Broglie Equation [ENDORSED]
Also, when you need to use De Broglie the problem will give you mass and/or velocity, whereas if you were to use the other equation relating wavelength to frequency, they would give you the frequency of the photon.
- Thu Oct 12, 2017 10:01 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Atomic Spectroscopy Post-Assessment Q28
- Replies: 2
- Views: 474
Atomic Spectroscopy Post-Assessment Q28
The question is: The meter was defined in 1963 as 1,650,763.73 wavelengths of radiation emitted by krypton-86 (it has since been redefined). What is the wavelength of this krypton-86 radiation? To what region of the electromagnetic spectrum does this wavelength correspond (i.e. infrared, ultraviolet...
- Wed Oct 11, 2017 8:46 pm
- Forum: Photoelectric Effect
- Topic: Photoelectric Effect Online Assessment Q34
- Replies: 1
- Views: 683
Photoelectric Effect Online Assessment Q34
The question is: B. If molybdenum is irradiated with 194 nm light, what is the maximum possible kinetic energy of the emitted electrons? So from part A, I got 7.22 x 10^-19 J as the threshold energy. For this question, I tried using the equation E = hv and then substituting v = c/wavelength and when...
- Tue Oct 10, 2017 9:45 pm
- Forum: Photoelectric Effect
- Topic: Photoelectric Effect: Post Module Assessment Q. 28, 29, and 30
- Replies: 12
- Views: 1455
Re: Photoelectric Effect: Post Module Assessment Q. 28, 29, and 30
For 28, what would you plug in for m in the equation E = 1/2mv^2? I am struggling with this one as well.
- Wed Oct 04, 2017 9:45 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Terminology Clarfication [ENDORSED]
- Replies: 7
- Views: 1609
Re: Terminology Clarfication [ENDORSED]
I think once we begin learning to name compounds this will make more sense. Typically, when you name compounds that contain a transition metal + a nonmetal, you must indicate the charge of the transition metal, since some have multiple charges. For example, Fe2O3 would be written as iron (III) oxide...
- Mon Oct 02, 2017 7:57 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Help with G13 Homework Problem
- Replies: 2
- Views: 353
Help with G13 Homework Problem
The question is: To prepare a fertilizer solution, a orist dilutes 1.0 L of 0.20 m NH4NO3(aq) by adding 3.0 L of water. The orist then adds 100. mL of the diluted solution to each plant. How many moles of nitrogen atoms will each plant receive? Solve this exercise without using a calculator. So far,...

