Search found 32 matches
- Thu Mar 15, 2018 5:26 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Activation Energy
- Replies: 1
- Views: 424
Re: Activation Energy
In an endothermic process, the potential energy of the products is higher than that of the reactants. Its reverse process will be exothermic. In the endothermic process, the reaction must build up activation energy that is even greater than the difference in the two potentials. On the other hand, th...
- Thu Mar 15, 2018 4:44 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Slow/Fast Step
- Replies: 2
- Views: 385
Re: Slow/Fast Step
The elementary slow step will be the one that reflects the rate law. Sometimes this is given directly in the slow step in terms of concentrations of reactants. In other cases you may have to use the pre-equilibrium method to substitute out a concentration of an intermediate.
- Thu Mar 15, 2018 4:36 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Max work and delta G
- Replies: 1
- Views: 329
Re: Max work and delta G
The work that we refer to in these cases is non-expansionary (no volume change). There is a mathematical derivation on this Chemistry Community post!
viewtopic.php?t=5141
viewtopic.php?t=5141
- Mon Mar 05, 2018 11:03 pm
- Forum: General Rate Laws
- Topic: 15.3
- Replies: 3
- Views: 502
Re: 15.3
Unique rate of reaction takes the form:
For the reaction aA -> bB + cC (where a, b, and c are stoichiometric coefficients),
Rate =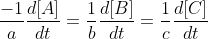
Hope this helps!
For the reaction aA -> bB + cC (where a, b, and c are stoichiometric coefficients),
Rate =
Hope this helps!
- Mon Mar 05, 2018 10:58 pm
- Forum: Balancing Redox Reactions
- Topic: Reducing Power
- Replies: 2
- Views: 426
Re: Reducing Power
Oftentimes, this is most easily done by looking at standard reduction potentials. The more negative the standard reduction potential, the less likely the element is to be reduced; this makes it more likely to be a reducing agent. Therefore, the more negative the standard reduction potential, the hig...
- Mon Mar 05, 2018 10:30 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Example 15.2
- Replies: 1
- Views: 301
Re: Example 15.2
Yes, you can plug in the data from any experiment to the general rate law to figure out k (once you have found the orders of each reactant).
- Wed Feb 28, 2018 11:17 pm
- Forum: Zero Order Reactions
- Topic: Real Life Example
- Replies: 2
- Views: 550
Re: Real Life Example
Zero order reactions are ones where reactant concentration does not affect reaction rate. As Dr. Lavelle said, this can happen when a catalyst or enzyme is needed for a reaction. When the catalyst/enzyme is saturated, an increase in concentration of reactant/substrate (reactants for enzymes) won't c...
- Wed Feb 28, 2018 11:01 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: When do we calculate for k
- Replies: 3
- Views: 493
Re: When do we calculate for k
You can calculate k using integrated rate laws or half life formulas if you are given information about the other variables that are involved (including final and initial concentrations and time/half life). Algebraically isolate k in the formula appropriate for the reactant order and solve.
- Wed Feb 28, 2018 10:57 pm
- Forum: Second Order Reactions
- Topic: 15.39
- Replies: 2
- Views: 419
Re: 15.39
The 0.19 moles/L of substance B is equated to 0.095 moles/L of substance A by stoichiometric ratios (1 mole A: 2 moles B). However, the 0.095 moles/L of substance A is the decrease in substance A, leaving 0.055 moles/L of substance A as the final concentration. The 0.37 is the ratio of final concent...
- Wed Feb 28, 2018 10:53 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: 15.17 a [ENDORSED]
- Replies: 3
- Views: 572
Re: 15.17 a [ENDORSED]
To find the order of each reactant, find trials where the concentration of only one of the reactants changes. Compare the change in reactant concentration with the change in rate. If the rate changes by the same factor as the concentration, it is order 1. If it changes by a square of the change in c...
- Tue Feb 20, 2018 9:38 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: 14.51
- Replies: 1
- Views: 310
Re: 14.51
E 0 must be zero in this case as the electrodes used on both sides are the same (Ag). The only thing that allows for the flow of electrons in the case of the same electrode would be a difference in concentration. Yet, in the standard case E is calculated when both sides are in standard 1M solution. ...
- Tue Feb 20, 2018 9:32 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 4.13 part b
- Replies: 1
- Views: 394
Re: 4.13 part b
Though I2 is given as a solid in the reaction, it isn't used as the electrode as it is limited in its ability to conduct electron flow. We use platinum, which is more conductive, for our electrodes in this case.
- Tue Feb 20, 2018 9:26 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Standard Hydrogen Electrode (SHE)?
- Replies: 1
- Views: 253
Re: Standard Hydrogen Electrode (SHE)?
A standard hydrogen electrode is one that is used as a reference for other electric potentials. By setting this as the baseline, we can find other standard potentials by checking the electron flow across a cell with hydrogen as one of the components and the substance in question as the other.
- Tue Feb 13, 2018 9:32 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bonds formed - negative?
- Replies: 3
- Views: 1745
Re: Bonds formed - negative?
Breaking bonds requires energy, so for broken bonds we use a positive bond enthalpy. On the other hand, forming bonds releases energy as it is a lower energy state; so we use negative bond enthalpies.
- Tue Feb 13, 2018 8:42 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Spontaniety
- Replies: 2
- Views: 504
Re: Spontaniety
No. Spontaneity does not say anything about speed. It is just about the Gibb's free energy. Speed has more to do with the kinetics of the reaction.
- Tue Feb 13, 2018 8:40 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Heat
- Replies: 1
- Views: 311
Re: Heat
Heat is transferred from hotter bodies to colder bodies and depends on the other thermodynamic phenomena that are occurring. In turn, it matters the path that the heat follows. Heat is not something that a body inherently has; it's defined as transferred energy, which depends on math.
- Fri Feb 09, 2018 3:52 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Delta G= Wmax
- Replies: 8
- Views: 2503
Re: Delta G= Wmax
W max refers to the maximum amount of work a system can do. Delta G is the maximum work that a system can do at a given temperature and pressure. So we can equate the two. I also found this previous post on Chemistry Community from Dr. Lavelle: https://lavelle.chem.ucla.edu/forum/viewtopic.php?t=5141
- Fri Feb 09, 2018 3:43 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Delta G Standard
- Replies: 2
- Views: 1077
Re: Delta G Standard
To address the second part of your question, standard ΔG refers to ΔG at 25 degrees C and 1 atm with 1M reactants and products. We know that standard ΔG= -RTln(K). Standard ΔG will provide information about the relative concentrations of products and reactants at equilibrium. It will only be equal t...
- Fri Feb 09, 2018 3:27 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: What is a Faraday? [ENDORSED]
- Replies: 3
- Views: 541
Re: What is a Faraday? [ENDORSED]
To add on to the previous reply, Faraday's constant is derived by dividing Avogadro's number (number of electrons in 1 mole) by the number of electrons that make up one Coulomb. This yields the 96485 C/mol from the previous response and also provides an explanation for its units.
- Thu Feb 01, 2018 9:22 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: 9.7
- Replies: 3
- Views: 442
Re: 9.7
This refers to page 281 in the textbook. The Cp=5/2 R refers to molar heat capacity at a constant pressure for atoms. This comes from Cp = Cv + R, as Cv for atoms is 3/2R. We can use the molar heat capacity for atoms of ideal gas in this problem as it plugs into ΔS = n*Cp*ln(T2/T1). This formula der...
- Thu Feb 01, 2018 8:48 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Homework 9.21
- Replies: 3
- Views: 433
Re: Homework 9.21
Since the molecules are all aligned in the same direction, the number of possible states is 1. In turn we use the formula (# of possible states)^# of particles. This yields 1^64 as there are 64 molecules.
- Thu Feb 01, 2018 6:30 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Negative delta s value
- Replies: 4
- Views: 11711
Re: Negative delta s value
A negative ΔS value will correspond to a spontaneous process when temperature is low and ΔH is negative. This is because of the relationship ΔG = ΔH - TΔS. We know that ΔG needs to be negative in order for the reaction to be spontaneous as that means that the entropy in the system is increasing (By ...
- Fri Jan 26, 2018 12:37 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: State properties of heat and enthalpy
- Replies: 2
- Views: 323
Re: State properties of heat and enthalpy
Heat transfer depends on the path in which the heat travels. Since we are talking about heat as a flow of energy, the path that this flow takes matters. This makes heat a path function. However, enthalpy, which is a change in a quantity of heat (absorbed/released), we ultimately only have to conside...
- Fri Jan 26, 2018 12:35 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Formula confusion [ENDORSED]
- Replies: 2
- Views: 191
Re: Formula confusion [ENDORSED]
S = Kb ln2 is just the formula for when the number of positions available doubles (W2 = 2*W1). We see this when volume doubles.
S = Kb lnW is just the general equation linking the degeneracy (number of accessible states) with entropy.
S = Kb lnW is just the general equation linking the degeneracy (number of accessible states) with entropy.
- Thu Jan 25, 2018 9:50 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Entropy of an Ideal Gas
- Replies: 2
- Views: 328
Re: Entropy of an Ideal Gas
Note that the formula in the previous reply (ΔS = n*R*ln(V2/V1)) applies when you plug in moles of gas (n). In a more general sense, ΔS = Kb*ln(W2/W1). When you multiply Kb by Avogadro's number, you end up with R. ΔS ends up q/T, where at a constant temperature, change in entropy is linked to a chan...
- Fri Jan 19, 2018 10:41 am
- Forum: Phase Changes & Related Calculations
- Topic: q water=-q ice equation
- Replies: 1
- Views: 171
Re: q water=-q ice equation
This is because the heat absorbed by the ice cube, which is colder than the water (heat is the transfer of energy due to a temperature difference: higher temperature to lower temperature), is exchanged from the water. The q value for the ice is positive because it absorbs heat, while the q value for...
- Fri Jan 19, 2018 10:23 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 8.29
- Replies: 4
- Views: 291
Re: 8.29
I agree with the previous replies: more bonds (greater molecular complexity) contributes to a higher heat capacity. To add to previous responses, this property stems from the fact larger, more complex molecules can move (vibrate, rotate, etc.) in more ways. More options for motion leads to more ener...
- Fri Jan 19, 2018 12:16 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Delta U?
- Replies: 7
- Views: 10662
Re: Delta U?
Just like the previous replies state, ΔU is the change in internal energy of the system. To add on to what the previous replies said, you can think of U as the kinetic energy + potential energy of the particles that your sample is comprised of. Internal energy is a state function, like enthalpy. It ...
- Fri Jan 19, 2018 12:10 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Enthalpy [ENDORSED]
- Replies: 3
- Views: 298
Re: Enthalpy [ENDORSED]
Additionally, it can be helpful to know that change in enthalpy (ΔH) is equal to q p or the heat given off/absorbed in a chemical reaction at a constant pressure. There is a derivation for this that comes from the equation: ΔH = ΔU + PΔV. ΔH = q p becomes useful in a lot of the exercises from the te...
- Fri Jan 12, 2018 1:19 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Catalysts and Enthalpies
- Replies: 2
- Views: 509
Re: Catalysts and Enthalpies
I agree! Catalysts would decrease the activation energy of a particular reaction, which could represent a different pathway in getting from your initial point to your final point. However, given enthalpy is a state function, altering the path does not alter the value of enthalpy, so enthalpy remains...
- Fri Jan 12, 2018 12:24 pm
- Forum: Phase Changes & Related Calculations
- Topic: ∆Hsub= ∆Hfus+ ∆Hvap
- Replies: 4
- Views: 3375
Re: ∆Hsub= ∆Hfus+ ∆Hvap
To add on to the previous replies, we know that state functions do not consider the path taken to get from point A to point B. In this case, even though the path for sublimation is different from the path where fusion is followed by vaporization, since the initial (solid) and final (vapor) states ar...
- Fri Jan 12, 2018 12:08 pm
- Forum: Phase Changes & Related Calculations
- Topic: Constant Temperature
- Replies: 4
- Views: 409
Re: Constant Temperature
One way to look at it is that the heat isn't contributing to the kinetic energy of the molecules, as the heat is going towards breaking intermolecular forces. Since average kinetic energy of the water molecules is unchanged when the phase change is happening, temperature stays constant. On the other...

