Search found 54 matches
- Sat Mar 17, 2018 1:31 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.73a - Resonance of Benzene
- Replies: 2
- Views: 436
8.73a - Resonance of Benzene
How do you know when to use the lewis structure of benzene with resonance between the 6 carbon bonds, versus using bond enthalpies of 3 single and 3 double carbon - carbon bonds?
- Thu Mar 15, 2018 3:09 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Coefficients in Rate Law
- Replies: 2
- Views: 426
Coefficients in Rate Law
What are the cases when we can use the molar coefficients in an equation as the order when writing the rate law, instead of the experimentally determined order? And what exactly allows us to assume that the coefficient is also the order?
- Thu Mar 15, 2018 2:57 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: How do you use a combined equilibrium equation?
- Replies: 1
- Views: 413
Re: How do you use a combined equilibrium equation?
Could you post the question that goes along with this question? Also, you do know T because the reaction happens at standard conditions, which is 298 K .
- Thu Mar 15, 2018 2:22 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Test #2 #7
- Replies: 5
- Views: 1442
Re: Test #2 #7
The two equations you use are 1: Cr2O7^2- + 14H+ + 6e- -> 2Cr^3+ +7H2O . E=1.33V 2: Cr3+ + 3e- -> Cr(s) . E=-.74V Notice that a sum of these after balancing the charges creates the overall equation. Since electric potential is not a state function, you have to use Gibbs Free Energy (delta G=-nFE) G(...
- Sun Mar 11, 2018 9:37 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: 15.71
- Replies: 1
- Views: 302
Re: 15.71
You basically solve this as you would a regular rate law with multiple steps. You cancel out the species/structures that are products in one equation and thus used as reactants in the others. These are your intermediates. The catalyst is not consumed in the reaction and is therefore present in the b...
- Sun Mar 11, 2018 9:30 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Homework Problem 15.67
- Replies: 1
- Views: 326
Re: Homework Problem 15.67
You have to find the proportion of rate of the catalyzed reaction to rate of the uncatalyzed reaction. The rate is equal to the two Arrhenius equations, k=A*exp(-Ea/R*T). The activation energy (Ea) of the catalyzed reaction is 75/ 125 times the activation of the uncatalyzed reaction, which is (.60)....
- Sun Mar 11, 2018 9:16 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: About the diagram of the transition state
- Replies: 1
- Views: 278
Re: About the diagram of the transition state
The main component of this diagram bust be that the two Br's must be next to each other and the NO must be ejected together. The NO is written together because that is how the gas is released and not the N and O separately in the case of decomposition.
- Sun Mar 04, 2018 9:39 pm
- Forum: Zero Order Reactions
- Topic: 15.15
- Replies: 3
- Views: 555
Re: 15.15
Zero order means that the rate of the reaction is independent of the concentration of the species.This is why it is disregarded in the rate law and therefore also in finding the order of A and B.
- Sat Mar 03, 2018 9:38 pm
- Forum: Second Order Reactions
- Topic: 15.19
- Replies: 2
- Views: 383
Re: 15.19
Using experiments 3 and 1, the concentration of A remains as 1.25 and the concentration of C remains as 1.25 also. The important thing to note here is not that both A and C have a concentration of 1.25 but that A's and C's concentrations stay consistent separately. 50.8/8.7 is 5.839 and the ratio of...
- Sat Mar 03, 2018 9:29 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: HW15.101 Regarding OH
- Replies: 2
- Views: 363
Re: HW15.101 Regarding OH
Typically, the rate law would come from the slow step so, R=k2 [HOCL][I-]. However, HOCL is an intermediate, so it needs to be expressed in terms of other products and reactants of the other fast reactions. Using the equation ClO-+H2O--->HClO+OH-, K = [HOCL][OH]/[OCL]. From this equilibrium equation...
- Sun Feb 25, 2018 8:32 pm
- Forum: General Rate Laws
- Topic: Rate Constants
- Replies: 4
- Views: 644
Re: Rate Constants
The rate constant changes with temperature because the rate of a reaction is dependent on temperature. This has to do with activation energy and the likeliness of a reaction happening because a greater presence of reactants (concentration) or a higher temperature, meaning more kinetic energy, which ...
- Sun Feb 25, 2018 7:06 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Electrochemical Series
- Replies: 3
- Views: 586
Re: Electrochemical Series
It is series of chemical elements arranged in order of their standard electrode potentials. It shows the order in which metals replace one another from their salts. Basically a list of reduction half- reactions.
- Sun Feb 25, 2018 7:04 pm
- Forum: *Free Energy of Activation vs Activation Energy
- Topic: What is Free Energy of Activation
- Replies: 11
- Views: 3612
Re: What is Free Energy of Activation
You can also think of it as energy difference between the transition state of a reaction and the ground state of the reactant or the difference between transition states and intermediates.
- Sun Feb 18, 2018 9:12 pm
- Forum: Balancing Redox Reactions
- Topic: "Rules"
- Replies: 7
- Views: 863
Re: "Rules"
You would add the H20 first to balance the oxygens, then balance hydrogens using H+, and after balancing the charges, convert the H+ to H20 by adding OH¯ to both sides. The side with the H+ will determine how many hydroxide to add.
- Sun Feb 18, 2018 8:47 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 14.13 (d)
- Replies: 2
- Views: 379
Re: 14.13 (d)
The equation must be reversed because the original oxidation reaction is Au3+(aq) + 3e- -> Au (s). This must be reversed into Au (s) -> Au3+(aq) + 3e- so that the Au 3+ ends up in the products side of the cell reaction given in the problem, Au3+ (aq) -> Au(s) + Au3+ (aq).
- Tue Feb 13, 2018 11:30 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Using the specific heat capacity ice
- Replies: 1
- Views: 403
Using the specific heat capacity ice
What would would be a scenario when to use the specific heat capacity of ice, instead the usual 4.184 for water?
- Sun Feb 11, 2018 6:40 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Derivation of Formulas
- Replies: 5
- Views: 806
Re: Derivation of Formulas
I don't think we have to derive them, but we should understand where the equations come from and exactly why and how, conceptually, we can manipulate equations in such ways.
- Fri Feb 09, 2018 10:21 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Homework Question 9.9
- Replies: 2
- Views: 542
Re: Homework Question 9.9
You only use 3/2 R if the ideal gas is at constant volume, which in this case it is not.
- Fri Feb 09, 2018 10:13 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: ΔG=0
- Replies: 2
- Views: 469
Re: ΔG=0
When delta G is zero, that means the system is at equilibrium and has no available energy. At boiling point it is zero because there is no change in temperature or pressure, therefore, by delta G = (delta H of vaporization) - (T of vaporization) X (delta S of vaporization) =0. It is also zero at the...
- Sun Feb 04, 2018 6:17 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 9.65
- Replies: 2
- Views: 331
Re: 9.65
You would calculate the entropy of formation of one mole of the substance. If 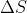 is positive, then the substance is more stable at higher temperatures. If it is negative, it is less stable.
is positive, then the substance is more stable at higher temperatures. If it is negative, it is less stable.
- Sun Feb 04, 2018 6:09 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Free Energy
- Replies: 2
- Views: 320
Re: Free Energy
The equations that basically sum up these relationships are  (o) = -RTlnK and G = G(o) = RTlnP
(o) = -RTlnK and G = G(o) = RTlnP
- Sun Feb 04, 2018 12:45 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: negative entropy [ENDORSED]
- Replies: 10
- Views: 5773
Re: negative entropy [ENDORSED]
The change in entropy can be negative, and when this is the case, you can think of it as a system becoming more ordered. An example would be of condensation, in the transition from a gas (high entropy state) to a liquid (low entropy state).
- Sun Jan 28, 2018 3:08 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.77
- Replies: 1
- Views: 271
Re: 8.77
Because the six resonance - stabilized bonds hold more energy (3108 kJ) compared to the 2880 kJ of energy in the isolated bonds, they are more difficult to break.
- Sun Jan 28, 2018 1:58 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Non Spontaneous Reaction vs Spontaneous Reverse Reaction
- Replies: 2
- Views: 1391
Re: Non Spontaneous Reaction vs Spontaneous Reverse Reaction
In the forward reaction if entropy was positive and enthalpy negative, the reaction is spontaneous at all temperatures. In the case of the same reaction, except the reverse, the reaction would have negative entropy and enthalpy positive, which is the most unfavorable set of conditions, meaning the r...
- Sun Jan 28, 2018 1:39 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Relationship between entropy and spontaneity
- Replies: 3
- Views: 1700
Re: Relationship between entropy and spontaneity
To add to the previous answer, the spontaneity, or Gibbs free energy depends on entropy and enthalpy. Positive entropy and negative enthalpy means the reaction is spontaneous at all temperatures. Positive entropy and enthalpy means the reaction is spontaneous at high temperatures. Negative entropy a...
- Sun Jan 21, 2018 4:39 pm
- Forum: Phase Changes & Related Calculations
- Topic: 8.9
- Replies: 2
- Views: 383
Re: 8.9
Use the equivalency of the ideal gas constants, 8.314 J K /mol = .08206 L atm / K mol . the - 1.48 is in L atm so through dimensional analysis of multiplying by 8.314 J K /mol over .08206 L atm / K mol , this should result in - 150 Joules.
- Sun Jan 21, 2018 4:17 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: 8.67
- Replies: 3
- Views: 370
Re: 8.67
The reaction for 1 mol of gaseous water is H2(g)+1/O2(g)->H20(g). The question asks for to find the enthalpy of formation of liquid water. This involves use of the equation: Enthalpy of formation of liquid water = Enthalpy of formation of gaseous water- Enthalpy of vaporization to account for the ph...
- Sun Jan 21, 2018 3:35 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard State of Elements
- Replies: 1
- Views: 373
Standard State of Elements
What are all the elements for which need to know the standard states of and that their enthalpy of formation of its most stable form is zero? (ex. O2, N2)
- Sun Jan 14, 2018 2:41 pm
- Forum: Calculating Work of Expansion
- Topic: Work equations
- Replies: 3
- Views: 310
Re: Work equations
The w=-nRTln(V2/V1) equation is used for reversible expansion. This means that the pressure is continually changing.
The w=-P*deltaV is sued for irreversible expansion. This is in situation with a constant external pressure.
The w=-P*deltaV is sued for irreversible expansion. This is in situation with a constant external pressure.
- Sun Jan 14, 2018 2:36 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Closed vs. Isolated
- Replies: 4
- Views: 613
Re: Closed vs. Isolated
A closed system allows heat transfer, but does not allow mass transfer. An isolated system allows neither heat nor mass transfer.
- Sun Jan 14, 2018 2:34 pm
- Forum: Calculating Work of Expansion
- Topic: Homework Problem 8.9
- Replies: 2
- Views: 439
Re: Homework Problem 8.9
Note that q is + 5.50 kJ as it is the amount of heat energy being gained. The work equation being used in the problem is w = -P(ext) x delta V. First convert the 750 torr to atm by dividing it by 760. Multiply the resulting .98684 atm of pressure by the change in Liters of volume (1.846-.345=1.501)....
- Sat Dec 09, 2017 12:40 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Delocalized Pi bonding
- Replies: 1
- Views: 738
Delocalized Pi bonding
What does it mean for a molecule to have delocalized pi bonding? How do we observe this given the structure?
- Thu Dec 07, 2017 9:15 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Percentage Deprotonation
- Replies: 4
- Views: 5712
Re: Percentage Deprotonation
Percentage deprotonation represents the ratio of the concentration of dissociated [H+] to the concentration of the undissociated [HA], or in other words the percentage of the initial acid that creates H3O+/H+ and it's conjugate base.
- Tue Dec 05, 2017 12:50 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Significant Figures in pH
- Replies: 1
- Views: 196
Significant Figures in pH
When rounding off significant figures for pH, do we start counting significant figures after the decimal point or the entire number. For example is ph= 5.42 2 significant figures or 3?
- Sun Dec 03, 2017 6:55 pm
- Forum: Lewis Acids & Bases
- Topic: Memorizing Acids and Bases
- Replies: 7
- Views: 897
Re: Memorizing Acids and Bases
I think it would be helpful to know for the final in order to know which method to use to find the pH, since strong acids completely disassociate and weak acids only partially disassociate.
- Sun Dec 03, 2017 6:22 pm
- Forum: Lewis Acids & Bases
- Topic: Bronsted vs Lewis
- Replies: 6
- Views: 818
Re: Bronsted vs Lewis
A Bronsted acid is a proton donor, and a Bronsted base is a proton acceptor. A Lewis acid is an electron acceptor, and a base is an electron donor.
- Sun Dec 03, 2017 4:16 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 11.89
- Replies: 4
- Views: 624
Re: 11.89
Although you are no explicitly told to ratio of reactants to products, you can deduce this information from the provided graph. Because use the C and B start from 0, you can assume that they are both the products. The A stats from a positive number, which means it was previously present, so it is th...
- Mon Nov 27, 2017 12:45 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Difference between molecular and electron geometry?
- Replies: 3
- Views: 55964
Re: Difference between molecular and electron geometry?
Electron geometry describes the arrangement of electron groups. Molecular geometry describes the arrangement of atoms, excluding lone pairs. For example, in the case of a trigonal planar shape as defined by electron geometry, there are three bonds. If atoms are bonded at all three locations, the mol...
- Mon Nov 27, 2017 12:33 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 4.11d
- Replies: 2
- Views: 456
Re: 4.11d
I think 107.5 is the exact measurement of the bond angle, whereas the use of "slightly less" means that you just estimate the bond angle through the electron placement and repulsion.
- Sun Nov 19, 2017 6:03 pm
- Forum: Lewis Structures
- Topic: Midterm Question: Lewis Structure for HOCO [ENDORSED]
- Replies: 6
- Views: 2234
Re: Midterm Question: Lewis Structure for HOCO [ENDORSED]
HOCO is the same order as how the elements are aligned in the lewis structure. First you count the number of valence electrons: 1 for Hydrogen, 2 x 6 for Oxygen, and 4 for Carbon. This equals 15 electrons total to be shared. Then begin by drawing a single bond between all the atoms, resulting in H-O...
- Sun Nov 19, 2017 12:42 pm
- Forum: Naming
- Topic: (H2O) or (OH2)? [ENDORSED]
- Replies: 3
- Views: 1801
Re: (H2O) or (OH2)? [ENDORSED]
In the case of coordination compounds, OH2 is preferred when the oxygen is what is bonding the metal central atom. In this case, the lone pairs on the O are the ones binding to the central atom to the left of the water molecule. H2O is preferred to show that the lone pairs on the O are binding to th...
- Sun Nov 12, 2017 7:40 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: T-shape vs. trigonal
- Replies: 2
- Views: 835
Re: T-shape vs. trigonal
A T shape has 3 bonds, and 2 lone pairs. An example of this is ClF3.
However, a trigonal planar shape has 3 bonds and 0 lone pairs. An example is BF3.
A trigonal pyramidal shape has 3 bonds and 1 lone pair like NH3.
I think the key difference here is the lone pairs.
However, a trigonal planar shape has 3 bonds and 0 lone pairs. An example is BF3.
A trigonal pyramidal shape has 3 bonds and 1 lone pair like NH3.
I think the key difference here is the lone pairs.
- Thu Nov 09, 2017 12:48 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Maximum electrons
- Replies: 2
- Views: 338
Re: Maximum electrons
You would count the total number of electrons in the s-, p-, d-, and f- subshells which is 32 electrons. Then, since the question is only asking for electrons with ms = +1/2, you would divide 32 in half to get your answer of 16 electrons. That is the answer I got also, but the answer from his old c...
- Thu Nov 09, 2017 11:35 am
- Forum: Quantum Numbers and The H-Atom
- Topic: Maximum electrons
- Replies: 2
- Views: 338
Maximum electrons
How would you go about counting the maximum number of electrons in atom with n=5 and ms=+1/2?
Would you only go up to the f subshell?
Would you only go up to the f subshell?
- Sun Nov 05, 2017 4:24 pm
- Forum: Ionic & Covalent Bonds
- Topic: #25 on Chapter 3
- Replies: 6
- Views: 992
Re: #25 on Chapter 3
Is there a reason I should know for why this rule works? The reason the criss-cross method outlined above works is because it balances charges. For example in magnesium Nitride, magnesium has a charge of 2+ and nitride 3-. Therefore, three magnesium ions are needed to balance out two nitrogen ions....
- Sun Nov 05, 2017 4:12 pm
- Forum: Trends in The Periodic Table
- Topic: Comparing Cations and Parent Atom Atomic Radius
- Replies: 2
- Views: 1201
Comparing Cations and Parent Atom Atomic Radius
I know that atomic radii decreases from left to right across a period as the effective atomic number increases, and they increase down a group as successive shells are occupied. Also that cations are smaller than their parent atoms and anions larger. However, when comparing for example, Cs+1 and Ba,...
- Sun Oct 29, 2017 5:45 pm
- Forum: Trends in The Periodic Table
- Topic: Ionization Energy [ENDORSED]
- Replies: 5
- Views: 767
Re: Ionization Energy [ENDORSED]
Is this the same answer to the question of why the second ionization energy of an atom is always higher than the first? Ionization energy is a measure of how difficult it is to remove an electron, so the second ionization energy is usually considerably larger, especially in the case of a Group 2 el...
- Sun Oct 29, 2017 1:38 pm
- Forum: Trends in The Periodic Table
- Topic: 2.60 [ENDORSED]
- Replies: 1
- Views: 427
Re: 2.60 [ENDORSED]
Atomic radii generally decrease from left to right across a period as the effective atomic number increases, and they increase down a group as successive shells are occupied. If the two ions are in the same group, the smaller ion will be the one that lies higher in the group, because its outermost e...
- Sun Oct 22, 2017 6:56 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Heisenberg Concept Question
- Replies: 2
- Views: 353
Re: Heisenberg Concept Question
This can be summed up through the photoelectric effect. An electron does not usually have enough energy to move up quantum states and orbitals on its own. It has to somehow be given the energy to do so, which we can observe directly through the experiment. The light gives energy to the electron and ...
- Sun Oct 22, 2017 6:28 pm
- Forum: DeBroglie Equation
- Topic: Exercise 1.42 [ENDORSED]
- Replies: 2
- Views: 488
Re: Exercise 1.42 [ENDORSED]
De Broglie's equation states that wavelength is equal to Planck's constant, h, divided by mass times velocity. To find the mass of helium for a single atom, you could divide the atomic mass, given in the periodic table, by Avogadro's number. 4.00 g/mol of Helium divided by 6.022 x 10^23 mol-1 is equ...
- Sun Oct 15, 2017 6:58 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Atomic Spectra Post Module #37
- Replies: 1
- Views: 249
Re: Atomic Spectra Post Module #37
The equation that would be used for finding the electronic energy levels is E=-hR/(n^2). The second part of the question is asking if the calculated number resulted from using this equation are going to correspond to real life experiments, of which mass spectroscopy is one of those experimental meth...
- Sun Oct 15, 2017 6:41 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Quantized Energy
- Replies: 5
- Views: 1046
Re: Quantized Energy
what exactly is the relationship between quantized energy and the n energy levels? I am a little confused In an atom, for example hydrogen, the electrons can only exist in specific energy states, which we associate with the "n energy levels", and they are quantized because they are set, t...
- Fri Oct 06, 2017 10:09 am
- Forum: Limiting Reactant Calculations
- Topic: M18 hELP!
- Replies: 3
- Views: 1365
Re: M18 hELP!
The first step to write a balanced equation. This would be H2A + 2XOH --> 2H20 +X2A Then with the given masses and molar masses, divide the mass by the molar mass for each of the reactants. For H2A : 1.20g/168gmol-1 = 0.00714 mol of H2A For XOH: 1.00g/125gmol-1 = 0.00800 mol of XOH Then you multiply...
- Thu Oct 05, 2017 10:43 pm
- Forum: Limiting Reactant Calculations
- Topic: Post/Pre Assessment Module Question
- Replies: 1
- Views: 290
Post/Pre Assessment Module Question
Just a heads-up to anyone going over the online module questions, For the question that asks, "According to the following equation, 0.750g of C6H9Cl3 is mixed with 1.000 kg of AgNO3 in a flask of water. A white solid, AgCl, completely precipitates out. What is the mass of AgCl produced?" T...

