Search found 50 matches
- Thu Mar 15, 2018 10:34 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Equations for different types of reactions
- Replies: 1
- Views: 400
Re: Equations for different types of reactions
For reversible, PV=nRT, and w=-nRTln(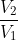 ). For irreversible, w=-P
). For irreversible, w=-P V.
V.
- Thu Mar 15, 2018 10:26 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 11.111
- Replies: 1
- Views: 247
Re: 11.111
In this homework problem, the second K is actually 10 times smaller than the first, so you would actually divide the first K by 10 rather than how you did 10 times the first K.
- Thu Mar 15, 2018 4:26 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Internal energy change = 0
- Replies: 2
- Views: 394
Re: Internal energy change = 0
The change in internal energy will be 0 for all isothermal expansions, whether the expansion is reversible or irreversible.
- Wed Mar 07, 2018 5:29 pm
- Forum: First Order Reactions
- Topic: First Order Integrated Rate Law
- Replies: 3
- Views: 453
Re: First Order Integrated Rate Law
They can both be used because ln of any number is equal to 2.303log of the same number.
- Wed Mar 07, 2018 5:13 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Diamond/Graphite
- Replies: 4
- Views: 6128
Re: Diamond/Graphite
According to thermodynamics, the reaction from diamond to graphite is spontaneous and favorable. However, because kinetics rather than thermodynamics is controlling this reaction, it occurs extremely slowly. So, diamond is kinetically stable, but thermodynamically unstable.
- Wed Mar 07, 2018 5:05 pm
- Forum: Zero Order Reactions
- Topic: Units of K based on the order
- Replies: 3
- Views: 1122
Re: Units of K based on the order
Because the rate of the reaction should have the units of mol/L s, for zero order, rate=k, so k has the units of mol/Ls. For first order, the units of k times concentration (mol/L) needs to be mol/L s, so the units of k are s^-1. For second order, the units of k times (mol/L)squared needs to be mol/...
- Fri Mar 02, 2018 12:57 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: 15.3 [ENDORSED]
- Replies: 7
- Views: 895
Re: 15.3 [ENDORSED]
In part c it is asking for the unique rate of the reaction, so you divide the rate for the certain term by its stoichiometric coefficient.
- Fri Mar 02, 2018 12:47 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 14.97
- Replies: 1
- Views: 360
Re: 14.97
Ka is the equilibrium constant for the dissociation of acids, so in this problem you would calculate it the same way you calculate regular k.
- Fri Mar 02, 2018 12:39 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Straight line
- Replies: 7
- Views: 1779
Re: Straight line
Also, if you plot a straight line with [A] vs t, it is zero order. ln[A] vs t means that it is first order, and 1/[A] vs t is second order.
- Fri Feb 23, 2018 1:09 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: ln vs log [ENDORSED]
- Replies: 5
- Views: 959
Re: ln vs log [ENDORSED]
Using log makes it easier to find the pH because pH= - log [H+].
- Fri Feb 23, 2018 1:06 pm
- Forum: First Order Reactions
- Topic: Reaction order
- Replies: 4
- Views: 619
Re: Reaction order
Also, the overall reaction order is found by adding together the reaction orders of the substances whose concentrations affect the reaction rate.
- Fri Feb 23, 2018 1:03 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 14.13d
- Replies: 3
- Views: 470
Re: 14.13d
This is what happens in a concentration cell.
- Wed Feb 14, 2018 1:49 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: What does E stand for?
- Replies: 3
- Views: 1994
Re: What does E stand for?
E is cell potential, which is an intensive property, and its units are in Volts, which is J/C.
- Wed Feb 14, 2018 1:41 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 9.47 Isothermal Irreversible Free Expansion
- Replies: 3
- Views: 592
Re: 9.47 Isothermal Irreversible Free Expansion
You would use )
- Wed Feb 14, 2018 1:38 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Difference between Gibbs Free Energy and standard Gibbs Free Energy
- Replies: 2
- Views: 6768
Re: Difference between Gibbs Free Energy and standard Gibbs Free Energy
Gibbs Free Energy is energy associated with chemical reactions and is equal to 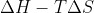 . Standard Gibbs Free Energy is when things are occurring at a standard state, which I believe should be 25 degrees C and 1 atm.
. Standard Gibbs Free Energy is when things are occurring at a standard state, which I believe should be 25 degrees C and 1 atm.
- Fri Feb 09, 2018 12:53 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: delta g
- Replies: 2
- Views: 349
Re: delta g
I think that Delta G is the free energy change of a reaction. Delta G r is the Gibbs Free Energy of the reactants and Delta G r knot is the standard free energy for the reactants.
- Fri Feb 09, 2018 12:47 pm
- Forum: Balancing Redox Reactions
- Topic: Anode vs cathode
- Replies: 6
- Views: 672
Re: Anode vs cathode
Also, the anode, oxidation half reaction, is always on the left, while the cathode, reduction half reaction, is always on the right.
- Fri Feb 09, 2018 12:44 pm
- Forum: Biological Examples (*DNA Structural Transitions, etc.)
- Topic: 9.73
- Replies: 2
- Views: 1145
Re: 9.73
- Wed Jan 31, 2018 5:04 pm
- Forum: Phase Changes & Related Calculations
- Topic: Example in Lecture
- Replies: 4
- Views: 522
Re: Example in Lecture
For this example, at 333K when deltaG=0, Br2(l) and Br2(g) are at equilibrium or boiling point, so if the temperature increases above 333K, the forward process would be favored and Br2 would vaporize to the gas phase only.
- Wed Jan 31, 2018 4:52 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Phase change question demonstrated in Lecture
- Replies: 4
- Views: 671
Re: Phase change question demonstrated in Lecture
When delta H is positive, it takes a very high temperature for entropy to dominate and cause delta G to become negative. So delta H + is only spontaneous at high temperatures.
- Wed Jan 31, 2018 4:29 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: State functions [ENDORSED]
- Replies: 7
- Views: 786
Re: State functions [ENDORSED]
Heat and work are not state functions because work is proportional to the distance traveled, meaning that the path taken does matter.
- Wed Jan 24, 2018 11:30 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Calorimetry
- Replies: 4
- Views: 524
Re: Calorimetry
It doesn't really matter if the change in temperatures are in Kelvin or Celsius because the Kelvin is in the same scale as Celsius, just +273, just make sure that the initial and final temperatures are both either in Kelvin or Celsius.
- Wed Jan 24, 2018 11:25 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Calculating heat (q)
- Replies: 4
- Views: 2170
Re: Calculating heat (q)
You would use q=mc(delta)T if you knew the mass of a substance and had the specific heat capacity while you would use q=nc(delta)T if you knew the number of moles and had the molar heat capacity because you want the final units of q to be in Joules.
- Wed Jan 24, 2018 11:17 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Entropy of a gas
- Replies: 2
- Views: 465
Re: Entropy of a gas
Entropy decreases when an ideal gas is compressed because the volume decreases and pressure increases.
- Wed Jan 17, 2018 4:15 pm
- Forum: Phase Changes & Related Calculations
- Topic: 8.91
- Replies: 1
- Views: 164
Re: 8.91
For 8.91, I first used q=mC \Delta T to find the amount of heat needed to raise the liquid water by 5°C, which was 0.312kJ. Then, I divided it by 0.5 hours to find the amount of heat transferred per hour, which was 0.624 kJ/hr. I multiplied that by 10.5 hours which was the time it took an ice cube t...
- Wed Jan 17, 2018 4:09 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: calculating specific heat
- Replies: 5
- Views: 828
Re: calculating specific heat
When using specific heat capacity to get q, only the mass and change in temperature are needed because the equation is q=mC \Delta T. The units for q is in joules and specific heat is in J/g°C, so when you multiply the specific heat by the mass (g) and change in temperature, (°C), you are left with ...
- Wed Jan 17, 2018 4:03 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Bomb Calorimeters
- Replies: 2
- Views: 546
Re: Bomb Calorimeters
Bomb Calorimeters are isolated systems as nothing exchanges with the surroundings because the bomb calorimeter is insulated and does not allow heat energy to transfer to the surroundings.
- Fri Jan 12, 2018 1:25 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: What type of system is a Bomb Calorimeter?
- Replies: 4
- Views: 2412
Re: What type of system is a Bomb Calorimeter?
Also, a Bomb Calorimeter is used during dconstant Volume calorimetry because the reaction chamber is a fixed volume.
- Thu Jan 11, 2018 3:53 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: #39 from Chapter 8
- Replies: 3
- Views: 305
Re: #39 from Chapter 8
You would use the specific heat capacity when you have the mass, while you would use molar heat capacity when you know the amount of moles because specific heat capacity measures the joules of heat needed to raise one gram of a substance by one degree C, while molar heat capacity measure the joules ...
- Thu Jan 11, 2018 3:40 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Most Stable Forms
- Replies: 4
- Views: 1083
Re: Most Stable Forms
An example of when the enthalpy of formation is 0 is when two Oxygen atoms form O2. This is because O2 is the natural form of oxygen.
- Thu Dec 07, 2017 12:54 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Relative Acidity Concept Question
- Replies: 4
- Views: 689
Re: Relative Acidity Concept Question
In the example given in class, hypochlorous acid was the most acidic out of HClO, HBrO, and HIO because CL has the highest electronegativity which stabilizes the negatively charged O by withdrawing electron density.
- Thu Dec 07, 2017 12:42 pm
- Forum: Amphoteric Compounds
- Topic: Amphoteric vs amphiprotic
- Replies: 2
- Views: 1721
Re: Amphoteric vs amphiprotic
Also, on the periodic table, there is a diagonal band of amphoteric oxides found between the metal oxides (bases) and nonmetal oxides (acids) that form amphoteric compounds.
- Thu Nov 30, 2017 10:42 am
- Forum: Lewis Structures
- Topic: 4.7A Homework
- Replies: 3
- Views: 1199
Re: 4.7A Homework
For SOCl2, the oxygen atom and two chlorine atoms are each bonded to sulfur by a single bond, and sulfur also has a lone pair to complete the octet. Then, because there are four areas of electron density with one lone pair, the shape is trigonal pyramidal.
- Thu Nov 30, 2017 10:39 am
- Forum: Lewis Structures
- Topic: 4.9
- Replies: 1
- Views: 460
Re: 4.9
Iodine has d-orbitals so it has an expanded octet and can accommodate more than 8 valence electrons. ICl3 is T-shaped because it has 5 areas of electron density with 2 lone pairs, and the Cl-I-Cl bond angle is less than 90 degrees because of the repulsion from the lone pairs.
- Wed Nov 22, 2017 3:54 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: T-shaped and Trigonal Pyramidal
- Replies: 6
- Views: 841
Re: T-shaped and Trigonal Pyramidal
T-shaped structures have 5 regions of electron density with 2 lone pairs, while Trigonal Pyramidal structures have 4 regions of election density with 1 lone pair.
- Wed Nov 22, 2017 3:33 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 4.9 Problem
- Replies: 4
- Views: 725
Re: 4.9 Problem
For ICl3, there are 5 regions of electron density, but only 3 are occupied by atoms, so there are two lone pairs, which means that it is T shaped.
- Fri Nov 17, 2017 6:51 pm
- Forum: Biological Examples
- Topic: Cobalt
- Replies: 4
- Views: 600
Re: Cobalt
The biological function of cobalt is that it is found in vitamin B12.
- Wed Nov 15, 2017 3:39 pm
- Forum: Hybridization
- Topic: Unhybridized Orbitals
- Replies: 2
- Views: 377
Re: Unhybridized Orbitals
Like in the example that was done in class with C2H4, the unhybridized 2p orbitals on each C atom overlapped side-to-side to form the pi bond.
- Wed Nov 08, 2017 12:44 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Beryllium Octet Rule
- Replies: 6
- Views: 10877
Re: Beryllium Octet Rule
The Boron in BF3 does not have a full octet because BF3 is a Lewis Acid, and the empty p-orbital on B can accept an electron lone pair from a Lewis Base.
- Wed Nov 08, 2017 12:35 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 4.11a
- Replies: 2
- Views: 350
Re: 4.11a
When the VSEPR formula is AX4E, the shape is always seesaw. The 5 pairs of electrons are arranged in trigonal bipyramidal, with 4 bonding pairs and 1 lone pair. Since only 4 of the 5 positions are occupied by atoms, they form the seesaw shape.
- Fri Nov 03, 2017 1:23 pm
- Forum: Ionic & Covalent Bonds
- Topic: Covalent/ Ionic Bond
- Replies: 5
- Views: 865
Re: Covalent/ Ionic Bond
Common examples of molecules with covalent bonds are HI, HBr, and HCl, whereas salts like KCL, KF, and LiF have ionic bonds.
- Fri Nov 03, 2017 12:50 pm
- Forum: Ionic & Covalent Bonds
- Topic: Ionic Bonds w/ Covalent Character
- Replies: 2
- Views: 480
Re: Ionic Bonds w/ Covalent Character
To add on, large, electron rich anions, can be highly distorted, so they have more covalent character, and are described as being highly polarizable. Small, highly charged cations, which have high polarizing power, cause large distortions and also lead to more covalent character.
- Thu Oct 26, 2017 9:43 pm
- Forum: Ionic & Covalent Bonds
- Topic: Ionic vs. Covalent Bonds [ENDORSED]
- Replies: 8
- Views: 1106
Re: Ionic vs. Covalent Bonds [ENDORSED]
So just to add on, in the case of KCl, K becomes a cation with a charge of +1 while Cl forms an anion with charge -1, so the attraction between the positive and negative charges causes them to form an ionic bond.
- Thu Oct 26, 2017 11:19 am
- Forum: Ionic & Covalent Bonds
- Topic: covalent bond
- Replies: 7
- Views: 1144
Re: covalent bond
It is true because non-metals do not form cations because their ionization energies are too high, so they share electrons to form covalent bonds.
- Thu Oct 19, 2017 11:12 am
- Forum: *Shrodinger Equation
- Topic: Electron Spin
- Replies: 6
- Views: 1157
Re: Electron Spin
Also, an electron that spins up and an electron that spins down can be paired in an orbital, while electrons with the same spin avoid each other and cannot be in the same orbital because no two electrons can have the same set of four quantum numbers.
- Thu Oct 19, 2017 10:56 am
- Forum: *Shrodinger Equation
- Topic: What is a node?
- Replies: 5
- Views: 745
Re: What is a node?
Also, at a node, the wavefunction is zero, so the electron density at the node is also zero, and the probability of finding an electron there is zero.
- Thu Oct 12, 2017 2:34 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Uncertainty in Kinetic Energy
- Replies: 2
- Views: 505
Re: Uncertainty in Kinetic Energy
Yes, you would use the equation E= mv2 and plug in
mv2 and plug in  v,which is the uncertainty for velocity, to get the uncertainty in kinetic energy.
v,which is the uncertainty for velocity, to get the uncertainty in kinetic energy.
- Wed Oct 11, 2017 4:53 pm
- Forum: Photoelectric Effect
- Topic: CH.1 #33 pt B
- Replies: 3
- Views: 557
Re: CH.1 #33 pt B
For part b, use 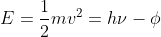 . Because you know the velocity of the ejected electron and the frequency of the radiation, you can solve for the work function, which is the energy required to remove the electron, and get 1.66 x 10-17 J.
. Because you know the velocity of the ejected electron and the frequency of the radiation, you can solve for the work function, which is the energy required to remove the electron, and get 1.66 x 10-17 J.
- Wed Oct 04, 2017 5:01 pm
- Forum: Empirical & Molecular Formulas
- Topic: L.39
- Replies: 5
- Views: 774
Re: L.39
After converting the amount of oxygen to moles (0.025mol), also convert the 1.50g of tin to moles, which is 0.0126 mol. Then because you have the amount of oxygen and tin in moles you can get the empirical formula because you know the ratio of tin to oxygen. You have Sn0.0126O0.025, and because 0.01...
- Mon Oct 02, 2017 4:49 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Finding the Concentration of a Specific Ion in Solutino [ENDORSED]
- Replies: 2
- Views: 2594
Re: Finding the Concentration of a Specific Ion in Solutino [ENDORSED]
For finding the concentration of potassium ions, first find the amount in moles of KCl, K2S, and K3PO4 in the solution. Then, split each compound and calculate the total amount in moles of potassium ions, which is 0.0229 moles. Then find the concentration of the potassium ions by dividing the number...

