Search found 60 matches
- Sat Mar 17, 2018 8:48 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Electron Transfer from Oxidation Number
- Replies: 1
- Views: 372
Electron Transfer from Oxidation Number
Can someone explain how we are able to figure out the number of electrons transferred in a redox reaction based just off the change in oxidation number? For example, if we are given the balanced equation C6H12O6 + 6O2 —> 6H2O + 6CO2, and that carbon’s oxidation number in glucose is 0, each H is +1, ...
- Sat Mar 17, 2018 11:15 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Preventing Charge Buildup
- Replies: 3
- Views: 605
Preventing Charge Buildup
What is the difference between using a porous disk or a salt bridge in a cell to prevent charge buildup? Do the two essentially accomplish the same thing, or are there any major differences we need to be aware of?
- Mon Mar 12, 2018 12:58 pm
- Forum: *Enzyme Kinetics
- Topic: Adsorption
- Replies: 13
- Views: 3190
Adsorption
What exactly is the difference between absorption and adsorption? How does adsorption work in relation to catalysts?
- Sat Mar 10, 2018 10:09 am
- Forum: General Rate Laws
- Topic: Fractional Rate Law [ENDORSED]
- Replies: 4
- Views: 1959
Fractional Rate Law [ENDORSED]
If we are given a rate law that involves a fraction of concentrations, such as 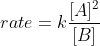 , do we still add the exponent values to find the overall order of the reaction? Like in this case, would the overall order be 2 + (-1) = 1? Thanks for clarifying!
, do we still add the exponent values to find the overall order of the reaction? Like in this case, would the overall order be 2 + (-1) = 1? Thanks for clarifying!
- Sat Mar 10, 2018 10:04 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Changes with Temperature [ENDORSED]
- Replies: 2
- Views: 341
Changes with Temperature [ENDORSED]
How exactly do we know that k, the rate constant, and K, the equilibrium constant, change with temperature? Is there a formula or relationship that relates these terms?
- Thu Mar 08, 2018 11:57 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Stoichiometric Coefficients
- Replies: 2
- Views: 535
Stoichiometric Coefficients
For the method of initial rates, do the stoichiometric coefficients of the balanced reaction equation matter in determining k or n?
- Thu Mar 08, 2018 11:54 pm
- Forum: General Rate Laws
- Topic: Problem 15.3
- Replies: 4
- Views: 786
Re: Problem 15.3
Equations for the rates of reaction are built off of the form Rate = \Delta Concentration / \Delta Time . That is why, for example, the differential rate law of a first-order reaction is -d[A]/dt . However, the order of reaction is not need for this problem, as you are given the change in concentrat...
- Sun Mar 04, 2018 2:32 pm
- Forum: General Rate Laws
- Topic: 2A --> B Rate Law
- Replies: 1
- Views: 2770
2A --> B Rate Law
In the kinetics information sheet on Dr. Lavelle's website, there is a formula given for the integrated rate law of a first-order reaction involving 2A --> B. The equation given is ln[A] = -2k't + ln[A] 0 . How was this equation obtained and why is it different than the simple A --> B reaction? When...
- Sun Mar 04, 2018 2:27 pm
- Forum: First Order Reactions
- Topic: Integrated rate law for first order reaction
- Replies: 3
- Views: 487
Re: Integrated rate law for first order reaction
I only know of one integrated rate law for first-order reactions. The integrated rate law, which is in linear form, is ln[A] t = -kt + ln[A] 0 . Plotting the natural log of concentration against time will produce a straight-line plot. I have only ever used and seen this one used, and as far as I kno...
- Wed Feb 28, 2018 9:15 pm
- Forum: Zero Order Reactions
- Topic: Units of k [ENDORSED]
- Replies: 13
- Views: 1558
Units of k [ENDORSED]
Can someone go over how we determine the units of k for different orders of reactions? What impacts do the units make on our calculations?
- Wed Feb 28, 2018 9:13 pm
- Forum: Zero Order Reactions
- Topic: Zero Order Reactions in Real Life [ENDORSED]
- Replies: 3
- Views: 1335
Re: Zero Order Reactions in Real Life [ENDORSED]
In addition, Dr. Lavelle mentioned that enzymes can become saturated. This means that all active sites on all the available catalysts are filled with a substrate. If this is the case, then increasing the concentration of the substrate will have no effect on the rate of catalyzation, because all acti...
- Thu Feb 22, 2018 6:38 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Inert Electrodes
- Replies: 4
- Views: 598
Inert Electrodes
The solutions manual mentions that inert electrodes are necessary for gas/ion electrode reactions. What are all possible situations where we would need an inert electrode? Would we be marked down if we included an inert electrode in the cell diagram where it wasn’t necessary?
- Tue Feb 20, 2018 11:07 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 14.11d
- Replies: 1
- Views: 256
Re: 14.11d
Figuring out why H+ was on the same side as O2 confused me at first too! However, when we attempt to balance the half-reaction for the anode, we can see that 5 H+ ions are needed on the left side to balance the number of hydrogen atoms on both sides. We now have 5 H+ on the left and 2H2O and H+ on t...
- Tue Feb 20, 2018 11:05 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 14.11 d
- Replies: 2
- Views: 286
Re: 14.11 d
This confused me at first too! However, when we attempt to balance the half-reaction for the anode, we can see that 5 H+ ions are needed on the left side to balance the number of hydrogen atoms on both sides. We now have 5 H+ on the left and 2H2O and H+ on the right. Because we have H+ on both sides...
- Tue Feb 20, 2018 11:00 pm
- Forum: Balancing Redox Reactions
- Topic: 14.5
- Replies: 1
- Views: 276
Re: 14.5
First off, just to clarify, the +5 isn't really a charge, it is an oxidation number. For the molecule BrO 3 - , we know that O typically has an oxidation number of -2. Because there are three oxygen atoms in the molecule, we can find that they collectively have an oxidation number of -6. We know tha...
- Tue Feb 20, 2018 10:56 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Electromotive Force
- Replies: 1
- Views: 309
Electromotive Force
What exactly is the electromotive force (emf)? What do we need to know about it in the context of this class, and why does it represent maximum potential difference?
- Sun Feb 18, 2018 7:32 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cathode and anode size
- Replies: 1
- Views: 227
Cathode and anode size
While doing a few practice problems, I came across a question that asked about "what would be seen at each electrode after some time." The solutions said that the cathode would become "bigger with time" while the anode would become "smaller with time". What exactly does...
- Sun Feb 18, 2018 11:54 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: w Max Equation
- Replies: 1
- Views: 2008
w Max Equation
Can someone explain how we can derive the wmax = -nFE equation? I understand that delta G equals maximum work, but I do not understand where the original equation comes from.
- Wed Feb 14, 2018 10:26 am
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Work
- Replies: 5
- Views: 802
Re: Work
Work done by expansion can be calculated by using w = -P * delta V. If delta V is zero, then there is no expansion occurring, and thus the overall work done is also zero. Note that under constant volume conditions, the change in internal energy of the system is given by q v and that the change in in...
- Wed Feb 14, 2018 10:20 am
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Zero Degeneracy
- Replies: 2
- Views: 491
Zero Degeneracy
Can degeneracy ever be zero? Why or why not?
- Sun Feb 11, 2018 2:02 am
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Degeneracy (W)
- Replies: 4
- Views: 522
Re: Degeneracy (W)
The number of ways of achieving a given energy state is called degeneracy, W. The relationship between W (degeneracy) and S (entropy) is the Boltzmann Equation: S = kb * ln(W)
- Sun Feb 11, 2018 1:53 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Pressure Change
- Replies: 1
- Views: 235
Pressure Change
Why do we put the intiial pressure term above the final pressure term in the equation that solves for change in entropy? In other entropy equations, final volume and final temperature are in the numerator, while initial volume and initial temperature are in the denominator.
- Sun Feb 11, 2018 1:32 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: "Ideal"
- Replies: 6
- Views: 774
Re: "Ideal"
Labeling the gases in a system as "ideal" means that the molecules occupy no space and that intermolecular attractions are negligible. We use the concepts of ideal gas laws to approximate the best-case scenario in a system. An ideal system does not have to be under conditions fo constant v...
- Sat Feb 03, 2018 8:02 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: G at minimum
- Replies: 6
- Views: 586
G at minimum
Why can there no longer be any change in a system when G (Gibbs free energy) is at a minimum?
- Sat Feb 03, 2018 7:56 pm
- Forum: Phase Changes & Related Calculations
- Topic: Degeneracy
- Replies: 4
- Views: 649
Re: Degeneracy
Degeneracy refers to microstates of the same energy. Looking at Dr. Lavelle's example with the two-sided flask from 01/22 lecture, we can see that the four microstates arise from the two particles going to two different sides of the flask while still at equivalent energy states. We assign degeneracy...
- Sat Feb 03, 2018 7:49 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Delta G [ENDORSED]
- Replies: 4
- Views: 521
Re: Delta G [ENDORSED]
Delta G, or the Gibbs Free Energy, tells us if the reaction occurs spontaneously or not. Because it is a calculated value from enthalpy and entropy, it actually doesn't always have to be negative. We know that spontaneous reactions occur when delta G is negative through the derivation Dr. Lavelle di...
- Sun Jan 28, 2018 1:16 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Internal Energy and Spontaneity
- Replies: 3
- Views: 342
Internal Energy and Spontaneity
When expanding the volume of a container full of gas in a vacuum, no work is done and so delta U is equal to zero. However, can someone explain why the gas expands spontaneously? Is it because of entropy, and why?
- Thu Jan 25, 2018 9:11 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Internal Energy in a Vacuum [ENDORSED]
- Replies: 5
- Views: 1000
Internal Energy in a Vacuum [ENDORSED]
Can someone explain why no work can be done through expansion in a vacuum? And why does this result in the values of both q and w to be zero?
- Thu Jan 25, 2018 9:07 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Out of Topic
- Replies: 3
- Views: 558
Re: Out of Topic
Dr. Lavelle's office hours can be found on the syllabus, Wednesday and Friday 2pm-3pm in Young Hall 3048A.
- Thu Jan 18, 2018 4:02 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.73a
- Replies: 3
- Views: 314
Re: 8.73a
Using bond enthalpies, we can calculate the enthalpy of the reaction. Breaking bonds requires energy (positive change) while creating bonds releases energy (negative change). Therefore, we can find the net energy change by adding all the bond enthalpies of the bonds broken, and subtracting from that...
- Thu Jan 18, 2018 3:56 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Extensive v Intensive Properties
- Replies: 2
- Views: 335
Re: Extensive v Intensive Properties
Heat capacity is the amount of heat required to change the temperature of an object by a certain amount. On the other hand, specific heat capacity is the amount of heat needed to raise the temperature of an object per unit mass of that object. We can see that heat capacity is an extensive property, ...
- Thu Jan 18, 2018 3:54 pm
- Forum: Phase Changes & Related Calculations
- Topic: 8.39
- Replies: 2
- Views: 199
Re: 8.39
Using the standard enthalpy of fusion when solving for the energy needed for state changes can be found by multiplying the number of moles by the standard enthalpy of fusion. Standard enthalpy of fusion is the amount of heat needed to melt 1 mole of substance, so multiplying that value by the number...
- Wed Jan 10, 2018 6:14 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: 2.29 parts b and d
- Replies: 4
- Views: 658
Re: 2.29 parts b and d
The right-most number in the previous user's table displays the maximum number of electrons that specific subshell can accommodate. In your question, the quantum numbers n=2 and l=1 tell us that we are looking at a 2p subshell. However, we do NOT know the orbital number (ml) or the spin number (ms),...
- Wed Jan 10, 2018 6:05 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Specific heat of water
- Replies: 3
- Views: 349
Re: Specific heat of water
Water has an exceptionally high heat capacity. This is caused by the weak hydrogen bonds among water molecules in the liquid form, constantly forming and breaking as the molecules slide past each other. In the gas form, water molecules are too far apart to interact like this. In the solid form, the ...
- Wed Jan 10, 2018 6:00 pm
- Forum: Phase Changes & Related Calculations
- Topic: Heat Capacity vs Specific Heat Capacity
- Replies: 3
- Views: 369
Re: Heat Capacity vs Specific Heat Capacity
Heat capacity is the amount of heat required to change the temperature of an object by a certain amount. On the other hand, specific heat capacity is the amount of heat needed to raise the temperature of an object per unit mass of that object. Another way to think about this difference is to see tha...
- Wed Jan 10, 2018 5:46 pm
- Forum: Phase Changes & Related Calculations
- Topic: Reaction in a Flask
- Replies: 5
- Views: 2173
Reaction in a Flask
I understand that if an exothermic reaction is occurring in a flask, energy is given off and thus the flask feels warm. However, can someone explain why an endothermic reaction occurring in a flask feels cool? I thought that endothermic reactions need to take in energy to occur.
- Mon Dec 04, 2017 10:16 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: EDTA
- Replies: 2
- Views: 534
Re: EDTA
EDTA has a charge of 4- but is still hexadentate because of the four charged oxygens and the lone pairs present on the two nitrogen atoms. The four single-bonded oxygen atoms with a negative charge will attach first to a central metal atom. It is also possible for the nitrogen lone pairs to bond and...
- Mon Dec 04, 2017 10:09 pm
- Forum: Naming
- Topic: Order of Chemicals/Ligands
- Replies: 1
- Views: 227
Re: Order of Chemicals/Ligands
When naming a coordination compound, you name the ligands of the overall molecule in alphabetical order, regardless of the prefix that tells you how many of the ligands are present. For example, if you have Cl- and (H2O)2 as ligands, you would still put diaqua first before chloro, because A comes be...
- Mon Dec 04, 2017 10:06 pm
- Forum: Conjugate Acids & Bases
- Topic: How to tell what the conjugate is easily?
- Replies: 1
- Views: 335
Re: How to tell what the conjugate is easily?
Remember that an acid reactant has a corresponding conjugate base product and that a base reactant has a corresponding conjugate acid product. The conjugates basically tell you what the role of the product is in the reverse reaction. For example, an acid that donates a proton will produce a conjugat...
- Tue Nov 28, 2017 1:13 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Conceptual Kc Problem
- Replies: 2
- Views: 222
Conceptual Kc Problem
For the reaction represented by the equation AgCl(s) <—> Ag+(aq) + Cl-(aq), is the equilibrium constant Kc >1, <1, or approx. =1? Explain your answer. My thinking was that because Kc = [Ag+][Cl-] and there is no denominator of reactant concentration because AgCl is solid, Kc would probably be greate...
- Tue Nov 28, 2017 1:02 pm
- Forum: Ideal Gases
- Topic: Kc to Kp Conversion
- Replies: 5
- Views: 939
Kc to Kp Conversion
Will we need to know how to convert values of Kc to values of Kp, and vice versa? If so, how do we do this? Do you need to use the Ideal Gas Law (PV=nRT)? There were a few problems like this on a worksheet my TA gave me, so I just wanted to clarify.
- Tue Nov 28, 2017 8:10 am
- Forum: Naming
- Topic: Problem 17.29
- Replies: 1
- Views: 304
Re: Problem 17.29
When naming coordination compounds, we need to look at the charge of the overall transition metal complex, or the stuff that's in the square brackets. If that complex has a negative charge, we need to add -ate to the end of the metal name. In part (b), the complex has 3+ charge, making it positive, ...
- Tue Nov 28, 2017 8:04 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium Constant vs. Reaction Quotient
- Replies: 1
- Views: 263
Re: Equilibrium Constant vs. Reaction Quotient
Q is our reaction quotient, while K is our equilibrium constant. K is a fixed ratio value for a reaction at equilibrium at a certain temperature, and can be found by calculating K=[P]/[R] using the equilibrium concentrations. On the other hand, Q is calculated the same way but for the concentrations...
- Mon Nov 20, 2017 11:58 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Ligands
- Replies: 2
- Views: 269
Re: Ligands
We can use the Lewis definition of acids and bases to explain the formation of coordination compounds. Ligands are Lewis bases, which means that they are electron pair donors. Metal cations are the centers of coordination spheres and are Lewis acids, or electron pair acceptors. The binding of Lewis ...
- Mon Nov 20, 2017 11:43 pm
- Forum: Naming
- Topic: bisoxalato
- Replies: 1
- Views: 464
Re: bisoxalato
Bisoxalato is referring to the (C2O4)2 in the coordination sphere. [C2O4]2- is the anion oxalate; because it is an anion, we need to add -o to the end of the anion's name when naming coordination compounds. So in this case, oxalate becomes oxalato. Also, the bis- prefix designates that oxalate is a ...
- Mon Nov 20, 2017 11:31 pm
- Forum: Biological Examples
- Topic: Ligands [ENDORSED]
- Replies: 3
- Views: 654
Re: Ligands [ENDORSED]
On Dr. Lavelle's Chem 14A website, there is a link labeled "Naming Coordination Compounds". This document contains common neutral and anionic ligands used in coordination compounds. The charges are listed as well. I think it would be best to familiarize yourself with these ions as they do ...
- Fri Nov 17, 2017 10:33 am
- Forum: Hybridization
- Topic: Coordination sphere
- Replies: 3
- Views: 585
Re: Coordination sphere
The coordination sphere encompasses the central metal cation and all the ligands attached around it. From this, we can also find the coordination number, which is essentially the number of ligands attached to, or number of bonds around, the central metal. We can usually find this information by look...
- Fri Nov 17, 2017 10:28 am
- Forum: Hybridization
- Topic: Filling the hybrid orbitals?
- Replies: 12
- Views: 1897
Filling the hybrid orbitals?
When we are drawing an Aufbau diagram for a hybridized orbital, should we fill those orbitals completely, with two spin-paired electrons in every orbital before putting electrons in the 2p orbital? Or should we place one electron in each hybrid orbital and p orbital (parallel spins throughout) befor...
- Thu Nov 09, 2017 8:32 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: 1.15
- Replies: 1
- Views: 231
Re: 1.15
The Lyman series of an atomic emission spectrum always results from an electron demotion from any principal energy level n to n=1. The Lyman series wavelengths also fall under the section of the electromagnetic spectrum that is UV light. Therefore, it is safe to assume that whenever UV light is give...
- Wed Nov 08, 2017 9:57 am
- Forum: Lewis Structures
- Topic: Lewis Acids and Bases
- Replies: 2
- Views: 438
Re: Lewis Acids and Bases
Coordinate covalent bonds are when one atom provides two of its electrons to share between the atoms involved in the covalent bond. The example given in lecture was the tetrafluoroborate anion, BF4-, in which boron was able to complete its octet because the fluorine ion donated two of its own electr...
- Sun Nov 05, 2017 5:11 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Lower in Energy? [ENDORSED]
- Replies: 6
- Views: 895
Lower in Energy? [ENDORSED]
When asked which orbital is lowest in energy, should we look at the principal quantum number (n) or angular momentum quantum number (l, determines whether s,p,d,etc subshell)? As in, would 3d be lower in energy, or would 4s be lower?
- Sun Nov 05, 2017 5:07 pm
- Forum: Trends in The Periodic Table
- Topic: Effective Nuclear Charge and Atomic Radius
- Replies: 1
- Views: 1214
Effective Nuclear Charge and Atomic Radius
Can someone please explain in-depth how effective nuclear charge (zeff) and atomic radius reate to each other? Why is it that when zeff increases, atomic radius decreases, and vice-versa? Thank you!
- Sun Oct 29, 2017 11:09 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: 2.29 parts b and d
- Replies: 4
- Views: 658
Re: 2.29 parts b and d
Each orbital in every subshell can hold at the most two electrons. The magnetic quantum number (ml) specifies a specific orbital within a subshell, and can have a value of -l <= ml <= +l. For example, within the p orbital (l=1) are the subshells ml= -1, 0, +1. In the given textbook problems, each va...
- Sun Oct 29, 2017 11:01 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: 2.33 and 2.1 [ENDORSED]
- Replies: 1
- Views: 253
Re: 2.33 and 2.1 [ENDORSED]
From what I understand, I think the atomc radius increases because the electron is getting arther away from the nucleus and thus does not feel the pull of the positively charged nucleus as strongly. The electron starts in the n=1 shell, as part of the 1s subshell. Once excited to the n=2 shell, or 2...
- Thu Oct 19, 2017 10:47 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Balmer and Lyman Wavelengths [ENDORSED]
- Replies: 4
- Views: 534
Balmer and Lyman Wavelengths [ENDORSED]
In the textbook, atomic spectroscopy was said to often result in spectral lines, some of which are grouped into specific series. For example, the Balmer series wavelengths represent visible light (electrons jump down to n=2) and the Lyman series wavelengths represent UV light (n=1). If in a problem ...
- Thu Oct 19, 2017 10:33 pm
- Forum: DeBroglie Equation
- Topic: Wave vs Particle Properties of Light
- Replies: 3
- Views: 601
Re: Wave vs Particle Properties of Light
In addition, electrons and therefore matter have both particle and wave properties. This duality is shown through the double-slit experiment, where the constructive and destructive interactions of waves resulted in diffraction patterns on different parts of the surface. However, these wavelike prope...
- Thu Oct 12, 2017 10:18 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Atomic Spectroscopy Post-Assessment Q28
- Replies: 2
- Views: 474
Re: Atomic Spectroscopy Post-Assessment Q28
So in this question, 1 meter is defined as 1,650,763.73 wavelengths of krypton radiation. So to find the wavelength, divide 1 meter by this number (1650763.73) to find the length of the wave, which turns out to be 606 nanometers, or 6.06 * 10^-7 meters. From there, you can just use E = (hc)/lambda a...
- Thu Oct 12, 2017 10:07 pm
- Forum: Properties of Electrons
- Topic: Energy of an Electron
- Replies: 2
- Views: 563
Re: Energy of an Electron
We are using E = 0 as a reference point in this case to represent the farthest an electron can be from its ground state. The negative sign basically means that an atomically bound electron has less energy than a free electron. Just remember that electrons in an atom do not ACTUALLY have negative ene...
- Thu Oct 05, 2017 11:36 pm
- Forum: Limiting Reactant Calculations
- Topic: Post/Pre Assessment Module Question
- Replies: 1
- Views: 291
Re: Post/Pre Assessment Module Question
Also, in questions involving mass percentages, it is important to thoroughly read the question to see which elements are involved and which respective percentages have been given in the question. For example, in a question that begins with "A compound of carbon, hydrogen, nitrogen, and oxygen h...
- Thu Oct 05, 2017 12:49 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Formula Unit [ENDORSED]
- Replies: 2
- Views: 427
Re: Formula Unit [ENDORSED]
From what I understand of formula units, I think of them as “ionic molecules”. They are the lowest whole number ratio of ions represented in an ionic lattice. For example, one Na+ ion and one Cl- ion (NaCl) is the formula unit for the ionic compound sodium chloride. For more information you can chec...

