Search found 54 matches
- Fri Mar 16, 2018 8:47 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: 9.1
- Replies: 5
- Views: 947
Re: 9.1
That is because the system is releasing heat, so it should be -q/t for the system, and positive q/t for the surroundings since they are absorbing heat.
- Fri Mar 16, 2018 8:46 pm
- Forum: *Enzyme Kinetics
- Topic: Kinetics and Enzymes
- Replies: 7
- Views: 1277
Re: Kinetics and Enzymes
I think we would need to know how enzymes can decrease the activation energy of a reaction. Therefore, if we were asked to find how the rate of the reaction, k, changes with or without the enzyme, we should be able to calculate it.
- Fri Mar 16, 2018 8:43 pm
- Forum: General Rate Laws
- Topic: Negative 1/a
- Replies: 8
- Views: 1115
Re: Negative 1/a
For example, if the question asked "what is the rate of decomposition of reactant A" the rate would be positive, even though the concentration of A is decreasing.
- Sat Mar 10, 2018 11:28 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: state functions
- Replies: 4
- Views: 926
Re: state functions
To illustrate with an example, work can't be a state function because it is proportional to the distance travelled or volume generated against an opposing force. So if you move an object from point A to B in a straight line, you do some work. But if you take a different, longer path that winds its w...
- Sat Mar 10, 2018 11:25 pm
- Forum: Balancing Redox Reactions
- Topic: Homework 14.13b
- Replies: 2
- Views: 366
Re: Homework 14.13b
You could also refer to this earlier post: viewtopic.php?f=140&t=19452.
- Sat Mar 10, 2018 11:24 pm
- Forum: Balancing Redox Reactions
- Topic: Homework 14.13b
- Replies: 2
- Views: 366
Re: Homework 14.13b
I think it is because I2 is not a good conductor, ie. because it is a nonmetal it would not make sense to use it in an electrochemical cell. Instead, platinum is a better element that would facilitate the reaction.
- Sat Mar 03, 2018 10:34 pm
- Forum: Zero Order Reactions
- Topic: Zero order common?? [ENDORSED]
- Replies: 5
- Views: 748
Re: Zero order common?? [ENDORSED]
Yeah I think it is possible to have a psuedo zero order reaction.
- Sat Mar 03, 2018 10:32 pm
- Forum: First Order Reactions
- Topic: Reaction Orders [ENDORSED]
- Replies: 2
- Views: 420
Re: Reaction Orders [ENDORSED]
Reaction orders are the sum of the order of each reactant. Each reactant's order tells us about how the concentration of that reactant affects the rate of formation of the products. For example, a zero-order reaction means that for a certain reactant, the concentration of it doesn't affect the rate ...
- Sat Mar 03, 2018 10:29 pm
- Forum: Second Order Reactions
- Topic: K in Rate Laws [ENDORSED]
- Replies: 2
- Views: 433
Re: K in Rate Laws [ENDORSED]
It depends on the derivation of the integrated rate laws. When you integrate the zero and first-order laws you get a -kt term, and when you integrate the second order law you get a positive kt term. In this case, where we relate these integrated laws to y = mx+b, m (the slope) is equal to positive o...
- Wed Feb 28, 2018 9:44 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3609840
Re: Post All Chemistry Jokes Here
What kind of fish is made of 2 Na atoms?
2Na.
2Na.
- Wed Feb 28, 2018 9:43 am
- Forum: General Rate Laws
- Topic: Negative 1/a
- Replies: 8
- Views: 1115
Re: Negative 1/a
Because the reactant is being consumed, the overall concentration of the reactant is decreasing so there is a negative rate of change. However, if the question asked at what rate was A being consumed, then it will be a positive answer. Just be careful about how the question is worded!
- Wed Feb 28, 2018 9:41 am
- Forum: General Rate Laws
- Topic: Negative in 15.5
- Replies: 3
- Views: 755
Re: Negative in 15.5
It's kind of like velocity, we prefer it to be positive, but depending on the wording it could be written as positive or negative.
- Wed Feb 28, 2018 9:38 am
- Forum: Administrative Questions and Class Announcements
- Topic: Kinetics Test Week 9
- Replies: 2
- Views: 482
Kinetics Test Week 9
What sections will be covered in the kinetics test next week and what topics are we expected to know?
- Sun Feb 18, 2018 8:36 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Isothermal, irreversible?
- Replies: 4
- Views: 820
Re: Isothermal, irreversible?
It only has to do with isothermal reactions not about reversibility or irreversibility.
- Sun Feb 18, 2018 8:29 pm
- Forum: Balancing Redox Reactions
- Topic: Balancing
- Replies: 3
- Views: 609
Re: Balancing
Yep that's correct!
- Sun Feb 18, 2018 8:02 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: State Functions
- Replies: 3
- Views: 506
Re: State Functions
State functions help with calculations because we find values for a process when we can't use the exact path described, but can use initial and final states to calculate state functions like enthalpy, entropy, etc.
- Sat Feb 10, 2018 9:05 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.85 Part C
- Replies: 1
- Views: 459
Re: 8.85 Part C
For every mole of N2 oxidized, the reaction absorbs 180.6kJ of heat. Therefore, if an unknown number of moles of N2 oxidized releases 492J, we can use divide this value by the molar enthalpy of this reaction (180.6kJ) to get the number of moles of N2 oxidized.
- Sat Feb 10, 2018 9:01 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: tested: gibbs free energy derivation?
- Replies: 1
- Views: 371
Re: tested: gibbs free energy derivation?
I heard one of the TAs said we should know how to derive the formulas for reversible/irreversible expansion work earlier this quarter. I don't think we have to know the specifics about deriving  G, but it would be helpful to know!
G, but it would be helpful to know!
- Sat Feb 10, 2018 8:57 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: delta H vs delta H naught
- Replies: 2
- Views: 1174
Re: delta H vs delta H naught
Yes that is correct, because 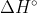 is the standard enthalpy for a mole of a given compound.
is the standard enthalpy for a mole of a given compound.
- Sun Feb 04, 2018 2:50 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3609840
Re: Post All Chemistry Jokes Here
Did you hear oxygen went on a date with potassium?
I heard it went OK.
I heard it went OK.
- Sun Feb 04, 2018 2:44 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: syllabus
- Replies: 4
- Views: 585
Re: syllabus
My best guess would be that Chapter 8, 9, 11 and part/or all of electrochemistry, depending on how much Dr. Lavelle is able to cover.
- Fri Feb 02, 2018 10:39 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Reversible systems
- Replies: 7
- Views: 912
Re: Reversible systems
I believe you would have to use both because you have to add the change in entropy associated with volume and the change in entropy associated with pressure to find the total entropy change of the system.
- Sat Jan 27, 2018 10:40 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: 9.7
- Replies: 1
- Views: 266
Re: 9.7
They come from the equipartition theorem (described in Chapter 8), but I do not think we are required to memorize them.
- Sat Jan 27, 2018 10:29 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Relationship between entropy and spontaneity
- Replies: 3
- Views: 1700
Re: Relationship between entropy and spontaneity
Not necessarily, as we learn later, Gibbs Free Energy or ( G) determines the spontaneity of a reaction. It is determined by change in enthalpy, absolute temperature, and entropy:
G) determines the spontaneity of a reaction. It is determined by change in enthalpy, absolute temperature, and entropy: 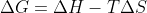 . If (
. If ( G) is negative, the reaction is spontaneous.
G) is negative, the reaction is spontaneous.
- Sat Jan 27, 2018 10:24 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Degeneracy (W) [ENDORSED]
- Replies: 8
- Views: 1270
Re: Degeneracy (W) [ENDORSED]
Degeneracy is the number of ways of achieving a given energy state, and is directly related to entropy using the equation Dr. Lavelle described in class with Boltzmann's constant. Gas has a higher degeneracy than a liquid or solid, because its molecules can exist in multiple states because the inter...
- Mon Jan 22, 2018 11:59 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: First Law Concept
- Replies: 4
- Views: 437
Re: First Law Concept
The first law talks about the conservation of energy, so therefore it makes sense that in an isolated system, the change in internal energy is 0. However, in open and closed systems, internal energy can change, because matter and energy or just energy can be exchanged between the system and its surr...
- Mon Jan 22, 2018 11:57 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Determining Enthalpy of Vaporization
- Replies: 4
- Views: 633
Re: Determining Enthalpy of Vaporization
I think it depends on the intermolecular forces of each molecule. If they are stronger, then it requires more energy to break those attractions to convert the substance from a liquid to a gas.
- Mon Jan 22, 2018 11:54 am
- Forum: Calculating Work of Expansion
- Topic: Integral Equation
- Replies: 2
- Views: 235
Re: Integral Equation
They represent the initial and final volume. In expansion work, the system is either being compressed or expanded, and these changes from initial to final volume are being represented by V1 or V2.
- Wed Jan 10, 2018 9:33 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: First Law and Calculating Final Temp (question 8.21)
- Replies: 3
- Views: 478
Re: First Law and Calculating Final Temp (question 8.21)
Yes, I believe the notation "heat lost by metal = -heat gained by water" is equivalent to q(metal) = -q(water).
- Wed Jan 10, 2018 9:25 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Most Stable Form for an Element
- Replies: 4
- Views: 648
Re: Most Stable Form for an Element
I think the most stable form of an element is the stablest configuration it can be found in, without it losing its purity. For example, Helium is most stable as a single atom, while Oxygen is most stable as a diatomic gas. Each of these are the stablest forms of their respective elements.
- Wed Jan 10, 2018 9:22 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Why does heat at constant pressure equal enthalpy?
- Replies: 1
- Views: 178
Why does heat at constant pressure equal enthalpy?
Why does the heat exchanged by a system and its environment at constant pressure equal  ? What happens when pressure changes, and how would that mathematically change how you calculate enthalpy?
? What happens when pressure changes, and how would that mathematically change how you calculate enthalpy?
- Thu Dec 07, 2017 2:42 pm
- Forum: Lewis Acids & Bases
- Topic: Self-Test 12.2A
- Replies: 1
- Views: 320
Re: Self-Test 12.2A
NO2- is the conjugate base of HNO2 (nitrous acid). It is a Lewis base because the lone pair on the nitrogen atom can be donated to a Lewis Acid. (The Lewis Structure for NO2- is a resonance structure with a single and double bond: O=N-O, and nitrogen has a lone pair)
- Thu Dec 07, 2017 2:38 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: 12.19; find change in pH for weak acids?
- Replies: 2
- Views: 451
Re: 12.19; find change in pH for weak acids?
No because this rule is only valid because strong acids completely disassociate in water. You would have to do the equilibrium calculations for a weak acid because not all of it disassociates.
- Wed Nov 29, 2017 2:48 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: O3
- Replies: 2
- Views: 424
Re: O3
O3 is polar and bent because there it has a resonance structure with one double bond and one single bond, so there is a dipole moment towards terminal single bonded O (it has a formal charge of -1). There is also a lone pair on the central atom giving it a bent shape.
- Wed Nov 29, 2017 2:45 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Calculating the Equilibrium Constant with Coefficients
- Replies: 2
- Views: 456
Calculating the Equilibrium Constant with Coefficients
Why do you raise each species in the equilibrium constant to its coefficient because when doing an ICE table you already add/subtract the change by the coefficient times x? For example, for 3H2 + N2 --> 2NH3, why do you raise the coefficent to each species after you already subtract or add by -3x or...
- Sat Nov 25, 2017 1:04 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: polydentate? 17.33
- Replies: 3
- Views: 328
Re: polydentate? 17.33
When you draw the lewis structure for CO32-, there is one C-O double bond, and two single C-O bonds. The single bonds leave the oxygen atom with a -1 formal charge and these atoms have the potential to bind a metal atom in two different places, hence making CO32- a bidentate ligand.
- Sat Nov 25, 2017 1:00 pm
- Forum: Hybridization
- Topic: Hybridization in Double and Triple bonds
- Replies: 2
- Views: 5978
Re: Hybridization in Double and Triple bonds
Hybridization is based on the regions of electron density around the central atom. Because a triple bond and a single bond would mean that the carbon is bound to 2 atoms, it has sp hybridization because of the two regions. It would have 2 electrons in the sp orbital for 2 sigma bonds, and 2 unpaired...
- Sat Nov 18, 2017 1:27 pm
- Forum: Naming
- Topic: Oxidation State vs. Formal Charge
- Replies: 3
- Views: 611
Oxidation State vs. Formal Charge
What's the difference between oxidation state and formal charge and when do we use one instead of the other?
- Sat Nov 18, 2017 1:25 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Expanded Octet Rule and Coordination Compounds
- Replies: 1
- Views: 530
Expanded Octet Rule and Coordination Compounds
I do not understand how nickel can be a central atom of a coordination compound and form 10 coordinate covalent bonds with other ligands. I thought Nickel is only allowed to have 18 valence electrons with an expanded octet (2 electrons from s, 6 from p, and 10 from d). Do the central atoms in coordi...
- Sat Nov 11, 2017 2:06 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Polar Bonds vs. Polar Molecules [ENDORSED]
- Replies: 5
- Views: 5990
Re: Polar Bonds vs. Polar Molecules [ENDORSED]
Polar bonds occur when the charge distribution between two atoms is unequal. For example, if two atoms do not share electrons equally, it is possible their bonds are polar. A polar molecule is one where the charge distribution is also asymmetric, so there are partial charges on the molecule.
- Sat Nov 11, 2017 2:02 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Isoelectronic [ENDORSED]
- Replies: 4
- Views: 1583
Re: Isoelectronic [ENDORSED]
No. F- gains 1 more valence electron and would have a complete octet, making it isoelectronic to the next noble gas (Ne). Be2+ and Li+ are isoelectronic to He, because they lose their 2s electrons.
- Sun Nov 05, 2017 9:47 pm
- Forum: Lewis Structures
- Topic: #37 on Chapter 3
- Replies: 2
- Views: 347
Re: #37 on Chapter 3
Alternatively, an easier method is to calculate the total number of electrons in the Lewis Structure. And then subtract the valence electrons of every known element, to find the mystery element's valence electrons (which should be 5). And Phosphorus is the only element in Period 3 with 5 valence ele...
- Thu Nov 02, 2017 2:55 pm
- Forum: Ionic & Covalent Bonds
- Topic: #25 on Chapter 3
- Replies: 6
- Views: 991
Re: #25 on Chapter 3
If you have ions of different charges, you can just switch their charges to make them coefficient. For example, in part C, where you have to write the chemical formula for indium(III) sulfide, Indium has a 3+ charge and Sulfur has a 2- charge. If you give the one element a coefficient that is the nu...
- Thu Nov 02, 2017 2:50 pm
- Forum: Lewis Structures
- Topic: #37 on Chapter 3
- Replies: 2
- Views: 347
Re: #37 on Chapter 3
Because the element is in Period 3 and has an expanded octet, you can deduce it will most probably be Silicon, Phosphorus, Sulfur or Chlorine. The other elements in Period 3 are more likely to form ionic bonds (Na and Mg), do not have an expanded octet (Al), or are not common in bonding reactions (A...
- Sat Oct 28, 2017 8:43 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: L [ENDORSED]
- Replies: 1
- Views: 264
Re: L [ENDORSED]
The quantum number, l, corresponds to the subshell ( 0 = s, 1 = p, 2 = d, 3 = f) and can be any value from 0 to n-1. For example, when n = 2, l can be 0 or 1. I don't think l can ever be equal to the value of n. What question are you referring to, in which l is equal to the value of n?
- Sat Oct 28, 2017 8:37 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Delocalization [ENDORSED]
- Replies: 3
- Views: 742
Re: Delocalization [ENDORSED]
Essentially, electron delocalization means that electrons are not confined inside the space of one bond. For example, in benzene, the electrons in the C-C bonds are not static, but rather they move around all C-C bonds because benzene displays hybrid resonance.
- Fri Oct 20, 2017 5:45 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: N Quantum Number [ENDORSED]
- Replies: 3
- Views: 529
Re: N Quantum Number [ENDORSED]
Yes the n quantum number represents the energy level the electron is at. It doesn't tell us orbital shape, orientation or the spin of the electrons. Therefore, n=2 can be 2s or 2p.
- Fri Oct 20, 2017 5:44 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: 3d And 4s Orbital Energy Levels [ENDORSED]
- Replies: 3
- Views: 1540
3d And 4s Orbital Energy Levels [ENDORSED]
How can 4s orbitals be lower than 3d orbitals in some cases, while in other cases they are not? For example, the electron configuration for Ca is [Ar]4s2, but for Sc, it is [Ar]3d1,4s2. Why is it not [Ar]4s2,3d1? I'm confused as to how 4s can have lower energy for some atoms, and higher energy for o...
- Fri Oct 20, 2017 1:07 am
- Forum: DeBroglie Equation
- Topic: Help with the concept of De Broglie's
- Replies: 8
- Views: 857
Re: Help with the concept of De Broglie's
If an object has a wavelength of less than x10^-15, then it still has wave-like properties, but they are basically undetectable. For example, if you throw a baseball, it will still have a wavelength, but it will oscillate at such a small wavelength, that it becomes irrelevant.
- Fri Oct 13, 2017 5:56 pm
- Forum: Properties of Electrons
- Topic: Stefan-Boltzmann law units
- Replies: 1
- Views: 318
Re: Stefan-Boltzmann law units
That's because the units have to cancel out so that both sides of the equation have the same units (W/m^2).
- Fri Oct 13, 2017 5:52 pm
- Forum: Photoelectric Effect
- Topic: Balmer vs Lyman [ENDORSED]
- Replies: 5
- Views: 841
Re: Balmer vs Lyman [ENDORSED]
No, you probably don't. You should probably know what energy levels they start at and what part of the electromagnetic radiation spectrum they are on. In the Lyman series, the relaxed state of the electron is n = 1 and it emits UV light. In the Balmer series, the relaxed state of the electron is n =...
- Thu Oct 05, 2017 4:32 pm
- Forum: Limiting Reactant Calculations
- Topic: M5
- Replies: 2
- Views: 749
Re: M5
The stoichiometric constants in a balanced chemical equation can be used to illustrate how much of each reactant you need to get a certain amount of product. I'm assuming that by getting part a, you know that ClO2 is the limiting reactant. Knowing that, you can use dimensional analysis to determine ...
- Thu Oct 05, 2017 4:27 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: E.27 [ENDORSED]
- Replies: 2
- Views: 458
Re: E.27 [ENDORSED]
In order to solve this problem, you would first find the mass of one mole of water, which is its molar mass (18g/mol). Because each mole of water contains 6.02x10^23 water molecules, you would divide the mass of one mole of water by Avogadro's number to find the mass one water molecule.
- Thu Oct 05, 2017 4:23 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: G25
- Replies: 4
- Views: 744
Re: G25
I think the method Ramya explained above does work and makes sense for G25, because it is not asking for when a specific number of molecules will be left in the dilutions. But it is important to understand the method with logarithms because if you look at G26, the problem might ask you to determine ...

