Search found 31 matches
- Thu Mar 15, 2018 7:45 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Slow/Fast Step
- Replies: 2
- Views: 385
Re: Slow/Fast Step
Yes, if we are given the rate law, we will be able to tell which step is the slow step, because the slow step is the limiting step that controls the rate of the reaction.
- Thu Mar 15, 2018 7:42 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: emf
- Replies: 3
- Views: 515
Re: emf
I agree with Alvin, emf stands for electromotive force, which is the same as E, the cell potential difference.
- Thu Mar 15, 2018 7:41 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Molecularity
- Replies: 2
- Views: 468
Re: Molecularity
Furthermore, from the molecularity chart we took notes on from lecture, you should also be able to write the corresponding rate law for the reaction.
- Thu Mar 08, 2018 1:19 pm
- Forum: First Order Reactions
- Topic: Equation variations
- Replies: 9
- Views: 1234
Re: Equation variations
Yes, the two equations you provided can be used interchangeably, as they are the same, just rearranged differently using logarithm rules. From the first equation you provided, we can rearrange it so that ln[A]_f - ln[A]_0 = -kt . Dividing by -1 gives us ln[A]_0 - ln[A]_f = kt . Using log rules, ln[A...
- Thu Mar 08, 2018 1:07 pm
- Forum: Zero Order Reactions
- Topic: zero order rate?
- Replies: 14
- Views: 1620
Re: zero order rate?
That is correct; a zero order reaction rate does not depend on the concentration of the reactant present, as long as there is some reactant. When integrating the rate law, we get Rate = 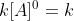 . Since anything raised to the zero power is 1, we can see that the rate just depends on k.
. Since anything raised to the zero power is 1, we can see that the rate just depends on k.
- Thu Mar 08, 2018 1:04 pm
- Forum: First Order Reactions
- Topic: Zero-order vs. First-order reaction graphs
- Replies: 5
- Views: 4191
Re: Zero-order vs. First-order reaction graphs
A zero order reaction graph is linear and has a negative slope when the y-axis is the concentration of the reactant, and a first order reaction graph is linear and has negative slope when the y-axis is ln [Reactant].
- Thu Mar 01, 2018 9:47 pm
- Forum: Zero Order Reactions
- Topic: Half-life of Zero Order [ENDORSED]
- Replies: 4
- Views: 679
Re: Half-life of Zero Order [ENDORSED]
Since Dr. Lavelle covered how to calculate half-life for zero, first, and second order reactions, I would probably study it and practice how to use it, even if the book doesn't require to do so, as I think anything he lectures on is fair game for the final.
- Thu Mar 01, 2018 9:44 pm
- Forum: First Order Reactions
- Topic: First Order Graph
- Replies: 11
- Views: 1969
Re: First Order Graph
For a first order graph, the ln [A] vs. time graph will be linear and decreasing, since the concentration of the reactant is decreasing. If the graph was changed to represent [A] versus time, then the graph would be a decreasing exponential function.
- Thu Mar 01, 2018 9:42 pm
- Forum: Second Order Reactions
- Topic: The graph of second order [ENDORSED]
- Replies: 5
- Views: 1813
Re: The graph of second order [ENDORSED]
After integrating the the rate law for a second order reaction, you can see that 1/[A] = kt+C, in which 1/[A] is on the y-axis and time (t) is the x-axis. Since the reactant is decreasing as the reaction progresses, [A] will get smaller and smaller meaning, \lim_{[A]\rightarrow 0} 1/[A] = \infty , a...
- Fri Feb 23, 2018 2:19 pm
- Forum: Balancing Redox Reactions
- Topic: Reaction E [ENDORSED]
- Replies: 5
- Views: 722
Re: Reaction E [ENDORSED]
E is an intensive property, meaning we do not need to manipulate it or multiply it by stoichiometric coefficients.
- Fri Feb 23, 2018 2:17 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagrams [ENDORSED]
- Replies: 7
- Views: 885
Re: Cell Diagrams [ENDORSED]
Inert electrodes like Pt are needed for some cells which don't have conducting ions. These electrodes allow for electron transfer without affecting the reaction.
- Fri Feb 23, 2018 2:16 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Finding n
- Replies: 15
- Views: 1908
Re: Finding n
n is the number of electrons transferred in the half reactions, but they need to be balanced first.
- Sun Feb 18, 2018 3:36 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 14.41(b)
- Replies: 1
- Views: 477
Re: 14.41(b)
Hey! I don't have the solutions manual so I can't see that it says n=1, but I did find a way to solve it using the same set up as you did. Using the Nernst equation, I got (\frac{0.0592}{2})log(\frac{pH3.0}{pH4.0}) . pH is a measure of H+, and we can use logs to find the H+ concentra...
- Fri Feb 16, 2018 4:31 pm
- Forum: General Science Questions
- Topic: Battery voltage
- Replies: 4
- Views: 876
Re: Battery voltage
Building off of Dylan's point, I'm not sure if this is the case in batteries, but often times, a voltage can be increased using step up transformers. I think Lavelle also pointed out that these batteries were very basic and rudimentary, and I'm sure batteries today are much more complex than the cel...
- Fri Feb 16, 2018 4:25 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Nernst Equation 2.303RT/F = .059V
- Replies: 4
- Views: 7902
Re: Nernst Equation 2.303RT/F = .059V
The 2.303 comes from the conversion from using logQ instead of lnQ. Either equation works and they're equivalent.
- Fri Feb 16, 2018 4:18 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Delta G standard
- Replies: 4
- Views: 693
Re: Delta G standard
I believe Lavelle mentioned in class that although it is Joules, it can also be seen as J/mol, so either way is fine.
- Fri Feb 09, 2018 12:07 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Conducting Metal
- Replies: 3
- Views: 427
Re: Conducting Metal
I think a conducting metal is required for electron transfer between two half reactions. In Dr. Lavelle's lecture on Wednesday, he stated that some half reactions have no conducting solids, so in one example platinum electrodes were used. Platinum works because it has no reaction with the solution a...
- Fri Feb 09, 2018 12:04 pm
- Forum: Balancing Redox Reactions
- Topic: Anode vs cathode
- Replies: 6
- Views: 675
Re: Anode vs cathode
I believe you are right. In a galvanic cell, the anode side is the oxidized side, as electrons flow out towards the cathode, or reduced, side. Therefore you would be right; the oxidation half reaction would be the anode side and the reduced half reaction would be the cathode side.
- Fri Feb 09, 2018 12:00 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode and cathode
- Replies: 9
- Views: 1445
Re: Anode and cathode
The anode side is the side that is oxidizes, meaning electrons are lost. The electrons move towards the cathode/reduced side.
- Fri Feb 02, 2018 3:28 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Isobaric [ENDORSED]
- Replies: 3
- Views: 577
Re: Isobaric [ENDORSED]
An isobaric process is one in which pressure is constant. The work done by or on the system can be calculated by taking the area under the line on the graph. The site below contains examples of what the graph looks like.
https://physics.info/pressure-volume/
Hope this helps
https://physics.info/pressure-volume/
Hope this helps
- Fri Feb 02, 2018 3:24 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Micro states
- Replies: 5
- Views: 653
Re: Micro states
The microstates are different positions, or states, that molecules can take in a system. An example talked about in class were the differences between solids and gases. Molecules in solids can have only few positions since molecules are packed together so tightly and can't move around. Thus, solids ...
- Fri Feb 02, 2018 3:03 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: How do we find out if a system is favorable? [ENDORSED]
- Replies: 7
- Views: 5029
Re: How do we find out if a system is favorable? [ENDORSED]
We also learned a new equations today in lecture, such as the Van't Hoff equation, which can be used to find the G of a reaction. When G is negative, the system is favorable, or spontaneous.
- Thu Jan 25, 2018 9:15 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Internal Energy in a Vacuum [ENDORSED]
- Replies: 5
- Views: 1005
Re: Internal Energy in a Vacuum [ENDORSED]
No work can be done through expansion in a vacuum because work is defined as -P*deltaV.
In a vacuum, there is no opposing force, so therefore the work done is 0. The book defines this as free expansion on page 264.
In a vacuum, there is no opposing force, so therefore the work done is 0. The book defines this as free expansion on page 264.
- Thu Jan 25, 2018 9:08 pm
- Forum: Calculating Work of Expansion
- Topic: Units
- Replies: 2
- Views: 1058
Re: Units
According to Table 8.1 on page 262 of the textbook, they note the standard convention of using units of Pascals (Pa) for pressure. However, Example 8.1 on page 264 gives a provides a good practice problem in which atm is used instead of pascals for pressure, and liters instead of meters cubed for vo...
- Thu Jan 25, 2018 9:03 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Formulas Under Different Conditions
- Replies: 2
- Views: 202
Re: Formulas Under Different Conditions
Building upon what Caroline said, you can also use the equation that Dr. Lavelle derived in class, which was -work= -nRTln(Vfinal/Vinitial).
This will give you the work, and since change in internal energy of the system is 0, q=-w
This will give you the work, and since change in internal energy of the system is 0, q=-w
- Wed Jan 17, 2018 5:13 pm
- Forum: Calculating Work of Expansion
- Topic: 8.17 Work done by a system
- Replies: 6
- Views: 702
Re: 8.17 Work done by a system
When a work is done by a system the work is considered negative because the system is losing energy. For instance, if we considered our bodies as the system, whenever we perform work, move, or exercise we use up some of our energy reserves, subtracting from our total energy. Since we are losing ener...
- Wed Jan 17, 2018 5:06 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: system and surroundings [ENDORSED]
- Replies: 3
- Views: 408
Re: system and surroundings [ENDORSED]
I agree with Michelle, since we are supplying heat to a copper kettle, we are adding heat to our system, not to our surroundings. I don't think it counts as a system, but rather the boundary of the outer boundary of the system.
- Wed Jan 17, 2018 5:04 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Heat Transfer at Constant Volume/Pressure
- Replies: 4
- Views: 531
Re: Heat Transfer at Constant Volume/Pressure
The way I learned this in the past during physics was by looking at the graphs. If you look at an isobaric system (pressure is constant), the work done by the system can be calculated by finding the area under the graph. If you look at an isochoric system (constant volume), you'll see that the line ...
- Wed Jan 10, 2018 5:07 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bond Enthalpies
- Replies: 5
- Views: 543
Re: Bond Enthalpies
The reactants have positive enthalpies because bonds are broken in the reactants, while bonds are formed in products. The breaking of bonds will always require some input of energy, meaning an addition of energy to the system. When bonds are formed, energy is released, meaning the system loses energ...
- Wed Jan 10, 2018 5:00 pm
- Forum: Phase Changes & Related Calculations
- Topic: Energy and Phase Changes
- Replies: 5
- Views: 577
Re: Energy and Phase Changes
Agreed, it might help to look at what molecules in solid, liquid, and gas phases look like. The main difference between solid and liquids is that molecules in liquids are still close but can move around past each other, while molecules in solids are close, compact, and cannot move around much. The d...
- Wed Jan 10, 2018 4:43 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Exothermic reaction in bond enthalpy example in lecture
- Replies: 5
- Views: 384
Re: Exothermic reaction in bond enthalpy example in lecture
Bonds being broken on the reactants side require energy, which is reported as a positive number. In this case, it was 978 kJ. On the products side, bonds are being formed, which release energy, reported as a negative number (-1086kJ). As a result, the difference between the two products was -59kJ, a...

