Search found 42 matches
- Sat Mar 17, 2018 9:59 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Reducing vs Oxidizing Agents
- Replies: 4
- Views: 745
Re: Reducing vs Oxidizing Agents
the way i think of it is first i find whats being reduced and whats being oxidized. Then to find the oxidizing or reducing agent i just reverse it, so the compound being oxidized is the reducing agent and the compound being reduced is the oxidizing agent.
- Sat Mar 17, 2018 9:57 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Pre equilibrium
- Replies: 5
- Views: 842
Re: Pre equilibrium
Yes!because they are equivalent
- Sat Mar 17, 2018 9:56 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Electrodes
- Replies: 3
- Views: 623
Re: Electrodes
You add Pt(s) as an electrode when the the half reaction does not have a solid metal for which the current can run through.
- Sun Mar 11, 2018 9:58 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Rate-determining slowest step
- Replies: 5
- Views: 1190
Re: Rate-determining slowest step
The slow step is usually given to you where there are different steps in a reaction mechanism. If not, the rate constant, k maybe given to you for you to identity the slow step. bottom line is, there is probably always going to be a way for you to determine the slow step of a reaction.
- Sun Mar 11, 2018 9:55 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Reading a Cell Diagram
- Replies: 4
- Views: 794
Re: Reading a Cell Diagram
You read a cell diagram from left to right. Usually the left reaction is the oxidized reaction (anode) while the right reaction is the reduced reaction (cathode)
- Sun Mar 11, 2018 9:47 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Buffer Solutions
- Replies: 2
- Views: 406
Re: Buffer Solutions
I dont think so. We've never explicitly covered buffer solutions in any of our lectures/discussions
- Sun Mar 11, 2018 9:45 pm
- Forum: First Order Reactions
- Topic: slope
- Replies: 3
- Views: 583
Re: slope
When you say linear plot of a first order reaction you are refering to the graph that results from a plot of ln[A] and time which results in a negative slope. However, if you were to graph [A] vs. time without the ln, you would not get a linear plot, instead a plot of exponential decay
- Sun Mar 11, 2018 9:43 pm
- Forum: Zero Order Reactions
- Topic: Definition of Reaction Rate
- Replies: 4
- Views: 751
Re: Definition of Reaction Rate
Because we are studying how concentrations change over time, the rate of a reaction are expressed in M (molarity) per seconds. or mol/L per seconds. Usually when masses are given they are accompanied by the volume of the reaction vessel which allows you to find the molarity.
- Sun Mar 11, 2018 9:41 pm
- Forum: General Rate Laws
- Topic: Rate Law sign
- Replies: 3
- Views: 457
Re: Rate Law sign
No a rate cannot be negative. A reaction cannot progress at -2 M/s.
- Sun Mar 04, 2018 4:58 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Rate Constant Units
- Replies: 9
- Views: 1338
Re: Rate Constant Units
The units of K must be able to cancel out with the units of the concentration in order for the rate units to always be M/s. so just add on however many M/s needed in order to get the rate to its proper units
- Sun Mar 04, 2018 4:56 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: slow step
- Replies: 3
- Views: 444
Re: slow step
The rate of the slow step is the rate of the full reaction as the reaction can only progress as fast as the slowest step
- Sun Mar 04, 2018 4:54 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Purpose of intermediate?
- Replies: 2
- Views: 373
Re: Purpose of intermediate?
Basically the intermediate step is there to explain how some reactants are converted to the products. Like in the example above, NO2 and CO cannot form NO and CO2. So the only explanation is that there is an intermediate reaction
- Sun Feb 25, 2018 9:29 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: anode vs cathode in non standard cell diagram
- Replies: 5
- Views: 737
Re: anode vs cathode in non standard cell diagram
Usually the anode is always on the left. If you aren't sure you can just look at both of the half reactions to determine which half reaction is the oxidation reaction and which is the reduction reaction.
- Sun Feb 25, 2018 9:27 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: calculating n
- Replies: 9
- Views: 1211
Re: calculating n
Yes you would work out the half reactions then balance the reactions out. The e- that results from the balanced half reaction will be the moles of e- you use for the equations.
- Sun Feb 25, 2018 9:20 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Units
- Replies: 7
- Views: 1201
Re: Units
I would use seconds unless otherwise stated simply because it is the SI units for time.
- Sun Feb 18, 2018 12:01 pm
- Forum: Balancing Redox Reactions
- Topic: Salt Bridge Versus Pourous Disk
- Replies: 6
- Views: 816
Re: Salt Bridge Versus Pourous Disk
Yes, it can transfer such ions like Na+ and Cl-
- Sun Feb 18, 2018 12:00 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: salt bridge
- Replies: 3
- Views: 726
Re: salt bridge
The purpose of the salt bridge is to balance out the charges by distributing the appropriate ions to the appropriate sides. By balancing out the charges on the anode and cathode sides, this allows continued movement of e- from the anode to the cathode
- Sun Feb 18, 2018 11:59 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Inert Conductor
- Replies: 3
- Views: 389
Re: Inert Conductor
Yes platinum is one of the common inert conductor
- Sat Feb 10, 2018 4:08 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: When to use + sign
- Replies: 11
- Views: 1062
Re: When to use + sign
Using + just makes it more identifiable that a system is gaining something. Its more clear than just leaving a number without a +
- Sat Feb 10, 2018 3:59 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode and cathode
- Replies: 9
- Views: 1428
Re: Anode and cathode
drawing the anode of the left is the conventional way. So I guess if you draw your cell correctly and get the direction of the e- current correct (from anode to cathode) it shouldn't matter if you draw the anode on the left or right. By recognizing which half rxn is the oxidation half or the reducti...
- Sat Feb 10, 2018 3:42 pm
- Forum: Balancing Redox Reactions
- Topic: Salt Bridges
- Replies: 14
- Views: 1354
Re: Salt Bridges
As the e- current transfers from the anode to the cathode, the ion in the anode loses the e- to the cathode making the anode gradually positive and the cathode gradually negative. Because of this, the cell will gradually stop receiving e- from the anode because it was already negative. The salt brid...
- Sat Jan 27, 2018 5:30 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Reversible isothermal expansion
- Replies: 4
- Views: 467
Re: Reversible isothermal expansion
I don't think it is necessary to know the derivations (like on past test in 14A we didn't need to know), but it's definitely helpful to know how you derive it because it can help understand the formula better.
- Sat Jan 27, 2018 5:27 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Entropy of gas vs liquid vs solid
- Replies: 7
- Views: 8900
Re: Entropy of gas vs liquid vs solid
By more "possible states" I think you mean more possible positions, as this determines the entropy of a substance. For a gas, it can occupy more possible positions, because like the person above said, gases can occupy a larger volume compared to liquids and solids. They are more able to fr...
- Sat Jan 27, 2018 5:23 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Formation of Snow in Clouds [ENDORSED]
- Replies: 5
- Views: 7431
Re: Formation of Snow in Clouds [ENDORSED]
Snow forming in clouds is basically the solidifying of liquid water to solid water. In liquid water, there is less bonding between the molecules compared to solid water. So in order for liquid water to solidify, the bonds need to form between the water molecules. And since we know that when bonding ...
- Sun Jan 21, 2018 2:18 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Homework problem 8.59
- Replies: 5
- Views: 2258
Re: Homework problem 8.59
Dr. Lavelle gave some of the common standard states for elements such as the diatomic elements like N2,O2 would be in the standard states if they were gases and others like Br2 would be standard states if it were in the liquid state. Other standard states he gave was solid Carbon as graphite.
- Sun Jan 21, 2018 2:11 pm
- Forum: Phase Changes & Related Calculations
- Topic: Calculating the Initial Temperature Of An Object
- Replies: 3
- Views: 1006
Re: Calculating the Initial Temperature Of An Object
use q=mC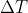
and plug the respective values into this:
-Qmetal=Qwater
and plug the respective values into this:
-Qmetal=Qwater
- Sun Jan 21, 2018 2:08 pm
- Forum: Calculating Work of Expansion
- Topic: Work done on / work done by system
- Replies: 5
- Views: 596
Re: Work done on / work done by system
Work done on the system means that the system is receiving the work so it is then written as +w. Work done by the system means the system is expending energy to do the work and so the work is -w.
- Sat Jan 13, 2018 11:17 am
- Forum: Phase Changes & Related Calculations
- Topic: Intensive vs. Extensive
- Replies: 4
- Views: 435
Intensive vs. Extensive
Can someone explain to me the difference between intensive and extensive variables, and when we should apply this to our thermochemistry/dynamics calculations?
- Sat Jan 13, 2018 11:15 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Specific heat capacity vs molar heat capacity
- Replies: 5
- Views: 618
Re: Specific heat capacity vs molar heat capacity
yes, its basically how must heat needed to raise one mole of a substance 1 degree, whether it is Celsius or Kelvin
- Sat Jan 13, 2018 11:14 am
- Forum: Phase Changes & Related Calculations
- Topic: Sublimation
- Replies: 4
- Views: 2463
Re: Sublimation
The triple point is exactly what it means: when all states of a substance exists at once. But like sublimation, it must occur in certain conditions
- Fri Dec 08, 2017 6:01 pm
- Forum: Bronsted Acids & Bases
- Topic: Determining acidity
- Replies: 3
- Views: 541
Re: Determining acidity
The reason why or acids are more read to lose e- is because usually the H proton is bonded to the oxygen. When the hydrogen is removed it results in a negative charge on the oxygen. In oxacids this negative charge on the oxygen is democratized from strong anions that pull the negative charge away fr...
- Fri Dec 01, 2017 9:04 pm
- Forum: Conjugate Acids & Bases
- Topic: Chemical Formula
- Replies: 5
- Views: 740
Re: Chemical Formula
A general rule is that if a molecule contains OH, it is considered a base. If a molecule has an H attached to it, it is generally an acid. Another way to identify if it is an acid or base is to identify the bronsted lowry acid and base by keeping track of the transfer of protons during the reaction.
- Fri Dec 01, 2017 9:02 pm
- Forum: Naming
- Topic: Order of Ligands
- Replies: 8
- Views: 864
Re: Order of Ligands
It doesn't matter if neutral / negative charge ligands go first or not. They just have to go alphbetically
- Sat Nov 18, 2017 11:15 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Ligands
- Replies: 1
- Views: 242
Re: Ligands
Ligands are molecules that donate a pair of electrons to an atom in order to form a coordination compound. They are also known as Lewis Bases, but in the biological world, they are referred to as ligands.
- Sat Nov 18, 2017 11:12 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Defining a Ligand
- Replies: 3
- Views: 327
Re: Defining a Ligand
The ligand is the molecule that donates a pair of electrons in a coordination compound. In other words, it is a lewis base.
- Sun Nov 12, 2017 11:32 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angle Exceptions
- Replies: 3
- Views: 1572
Re: Bond Angle Exceptions
When it gets to bond angles for structures that include lone pairs, we just have to know that the bond angle will be less than that of the expected one. For example, for a trigonal pyramidal, we only need to know that the bond angle that results from it, will be less than 109.5. This is because it w...
- Sun Nov 12, 2017 11:14 am
- Forum: Coordinate Covalent Bonds
- Topic: Ionic and Covalent Bonds
- Replies: 2
- Views: 457
Re: Ionic and Covalent Bonds
In addition to the answer above, you can also tell whether or not a bond is ionic or covalent by using the electronegativity guidelines that Dr. Lavelle gave us. If the electronegativity difference is greater than 2 then the bond is considered ionic. If the electronegativity difference is less than ...
- Sun Nov 05, 2017 2:35 pm
- Forum: Lewis Structures
- Topic: 3.51
- Replies: 3
- Views: 339
Re: 3.51
In addition to Scott's answer, one of the primary reasons why your lewis structure is not correct compared to the answer is that if you put Cl in the middle, it takes on 10 e-, which is not possible for it to do, since it goes over its octet. Hope this helps!
- Sun Nov 05, 2017 2:27 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Which one do I draw?
- Replies: 5
- Views: 768
Re: Which one do I draw?
Hi, Im pretty sure that they question will ask you. Such as: "Write the Lewis Dot Structure for "certain molecule". And if they require a VSEPR model, the question would say something along the lines of: "what shape does the molecule take according to the VSEPR model" or som...
- Sat Oct 28, 2017 8:36 pm
- Forum: Lewis Structures
- Topic: drawing Lewis structures [ENDORSED]
- Replies: 2
- Views: 433
Re: drawing Lewis structures [ENDORSED]
Like Sammy said, putting the atom with lowest ionization energy makes it easier to draw a symmetrical molecule, but I also I think since the atom in the center has the lowest ionization energy of all atoms in the molecule, it would be easier for that electron to share the e- because there is a small...
- Sat Oct 28, 2017 8:29 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Delocalization [ENDORSED]
- Replies: 3
- Views: 742
Delocalization [ENDORSED]
What does "delocalization" of electrons mean? I know that they have to do with resonance, but I'm having trouble trying to figure out what it actually means in context. If someone could explain it to me in a simpler way I'd appreciate it. Thanks!
- Sun Oct 15, 2017 3:41 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Heisenberg Uncertainty Equation
- Replies: 7
- Views: 748
Heisenberg Uncertainty Equation
I know we haven't gone over this in class yet, but I'm trying to figure this out. Can someone explain to me why this is statement is true? "You should expect the uncertainty in the position of an object as heavy as a marble to be very small, but the uncertainty in the speed of an electron, whic...

