Search found 53 matches
- Sat Mar 17, 2018 11:46 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Standard Gibbs Free Energy for Vaporization of Water
- Replies: 2
- Views: 1940
Re: Standard Gibbs Free Energy for Vaporization of Water
It should be 0 at 100C (373K) because it is the boiling point, but if you actually calculate using the values at the back of the book, you'll get -0.37kJ/mol. 0 is the correct one. We get a slightly different answer because the values at the back do not completely correspond with temperature. If we ...
- Sat Mar 17, 2018 11:42 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Adiabatic vs Isothermal
- Replies: 5
- Views: 2332
Re: Adiabatic vs Isothermal
Adiabatic: heat does not leave or enter system. This means that energy released as heat will not leave the system, but be used up as work (ex. gas expansion against a piston).
Isothermal: change in temperature (heat =/= temperature) is 0. This means that heat, or q, will be 0 (q=mCdeltaT)
Isothermal: change in temperature (heat =/= temperature) is 0. This means that heat, or q, will be 0 (q=mCdeltaT)
- Sat Mar 17, 2018 11:38 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: 9.5
- Replies: 4
- Views: 645
Re: 9.5
It is a transfer of heat, so thinking back to our ice cube in water problems, one will be positive and the other negative.
- Sat Mar 17, 2018 10:17 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Kinetically controlled reactions
- Replies: 3
- Views: 1134
Kinetically controlled reactions
How can we tell from a free energy diagram whether a reaction is kinetically or thermodynamically controlled? My thought process is that if that if the curve is really tall, it's more responsive to temperature, and therefore be thermodynamically controlled, but I saw a worksheet that said otherwise.
- Sat Mar 17, 2018 9:12 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3616124
Re: Post All Chemistry Jokes Here
I told my friends my chemistry jokes. There was no reaction other than the room growing cold.
Guess it wasn't spontaneous enough. Or maybe there was an exothermic one...
Guess it wasn't spontaneous enough. Or maybe there was an exothermic one...
- Sat Mar 17, 2018 8:53 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3616124
Re: Post All Chemistry Jokes Here
As a specialist in alchemy, Dumbledore decided to name his resistance movement against Voldemort inspired by his chemistry roots. As a result... The Order of the Phoenix Reaction, featuring characters like Sirius Black the intermediate who comes up but shortly dies, Ron Weasley the rate determining ...
- Sat Mar 17, 2018 8:36 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3616124
Re: Post All Chemistry Jokes Here
A chemistry student walks into a bar (err that serves non alcoholic drinks of course) and sees an attractive server. The student goes up to the server in an attempt to get the mixer to leave their shift. The student says:, If 'U minus work', we could make some heat. (ayyyy get it cause deltaU=q+w) (...
- Sat Mar 17, 2018 8:23 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3616124
Re: Post All Chemistry Jokes Here
Why do chemists always choose to turn into cats when given the ability to become an animal?
So they can say with finality: I mCAT
(I'm awful at these but they're so fun to make)
So they can say with finality: I mCAT
(I'm awful at these but they're so fun to make)
- Sat Mar 17, 2018 4:57 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: First Law Thermo
- Replies: 4
- Views: 718
Re: First Law Thermo
q(surr) = -q(sys) is correct, but it is not another accurate explanation of the rate law because we'd be ignoring the work component in internal energy.
- Sat Mar 17, 2018 4:26 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Isolated Systems
- Replies: 3
- Views: 498
Re: Isolated Systems
I don't think so because a piston in an isothermal chamber could allow for change in volume while still insulating it. So any changes in energy like a reaction releasing heat would be used up by the system as expansion work, for example.
- Sat Mar 17, 2018 4:22 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: isothermal = no internal energy change
- Replies: 4
- Views: 786
isothermal = no internal energy change
If there is no change in temperature/system is isothermal, does it imply no change in internal energy? Since deltaU=q+w and q=mCdeltaT, I get that q=0 if change in temperature is 0. I don't understand how w=0 as well though.
- Fri Mar 16, 2018 10:43 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Topics on final-- lattice enthalpy?
- Replies: 1
- Views: 354
Topics on final-- lattice enthalpy?
Do we have to know lattice enthalpy for the final? I've seen it pop up on some discussions here, but I can't remember doing any problems on it.
- Fri Mar 16, 2018 10:27 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.67b-- figuring out the chemical equation
- Replies: 1
- Views: 367
8.67b-- figuring out the chemical equation
8.67b calls for estimating the enthalpy of formation for methanol (CH3OH) in the liquid state. I understand what I'm supposed to do, but do not know how I'm supposed to find the chemical equation for methanol. I tried combustion, but realized that it wouldn't work. I checked the solutions and it lis...
- Fri Mar 16, 2018 10:22 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.67
- Replies: 3
- Views: 538
Re: 8.67
deltaH(vap) gives you the heat for a substance going from liquid to gas (vaporization). In this case, we are going from gas to liquid, so we'll reverse the sign. Then we add the two deltaHs. Think back to Hess's law problems-- we would make a positive enthalpy value negative (or vice versa) when we ...
- Fri Mar 16, 2018 9:15 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: 2nd law
- Replies: 2
- Views: 539
Re: 2nd law
Seconding the above answer. As long as you show your understanding that entropy is increasing in the universe I think it'll be fine.
- Fri Mar 16, 2018 8:59 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Split of questions on the final
- Replies: 3
- Views: 667
Re: Split of questions on the final
I don't think it was ever mentioned, but going off of what I heard from past finals and the 14A final, it'll be a relatively even split. My TA did mention that the stuff we went over recently (kinetics) will probably be a bit more challenging.
- Fri Mar 16, 2018 8:58 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: 8.51b
- Replies: 1
- Views: 344
8.51b
In 8.51, I calculated the heat released by the reaction for 1.4g of CO, but I don't understand why the solution( heat released for 1.0 mol of CO) is the same since the previous enthalpy value was for 1.4g of CO. Is it cause the solution is already in kJ/mol? Thanks.
- Mon Mar 12, 2018 7:06 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: 8.18
- Replies: 1
- Views: 290
8.18
Can anyone affirm or correct my thought process for 8.18? It's not an assigned problem, but I figured it's good practice. For a: since change in energy is + and volume appears to remain constant (w=0), so q must be + (heat absorbed). For b: change in volume is + (Vf>Vi) so work is -. I'm not certain...
- Mon Mar 12, 2018 4:59 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3616124
Re: Post All Chemistry Jokes Here
If only work were that simple (ft. 30sec MS paint)
- Mon Mar 12, 2018 4:53 pm
- Forum: Calculating Work of Expansion
- Topic: different types of work
- Replies: 4
- Views: 464
Re: different types of work
I don't think so since the syllabus didn't include Box 8.1 which lists the varieties of work. Conceptually, work isn't all that different for all the types listed though (raising a weight --> lifting something is the distance component and gravity is the Pex; extension--> the tension is the Pex and ...
- Mon Mar 12, 2018 4:41 pm
- Forum: Calculating Work of Expansion
- Topic: irreversible work [ENDORSED]
- Replies: 3
- Views: 512
Re: irreversible work [ENDORSED]
Conceptually, why does reversible expansion do more work. I know the curves but I don't understand why they are like that (the reversible curve makes sense since each change in Pex has a corresponding V, but the irreversible one confuses me).
- Mon Mar 12, 2018 4:13 pm
- Forum: Student Social/Study Group
- Topic: Chemistry Joke
- Replies: 57
- Views: 5290
Re: Chemistry Joke
What did the chemist buy from the beaver hunter?
Aldehydes
...sorry. I'm bad at these.
Aldehydes
...sorry. I'm bad at these.
- Thu Feb 22, 2018 1:24 am
- Forum: Balancing Redox Reactions
- Topic: 14.11
- Replies: 3
- Views: 701
Re: 14.11
Pt (Platinum) can be ignored because it's not losing or gaining electrons. It's acting as an inert electrode for electron transfer. You'll see Pt/inert electrodes pop up when both the oxidized and reduced species are in the solution OR it is a gas/ion electrode reaction.
- Thu Feb 22, 2018 1:21 am
- Forum: Balancing Redox Reactions
- Topic: 14.15a
- Replies: 3
- Views: 1661
14.15a
I'm really confused on how to find the half reactions when given: AgBr(s) --> Ag+(aq) + Br-(aq), a solubility equilibrium. I understand what happens at a cathode (reduction) and anode (oxidation). I thought that the half reactions would we somewhere along the lines of: cathode: Ag+ + e- --> Ag(s) an...
- Mon Feb 05, 2018 1:08 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Midterm
- Replies: 6
- Views: 837
Re: Midterm
I think there was a post somewhere on Chemistry Community that the derivations of the equations are fair game. I doubt it'll be tested on, though, since it's been stressed multiple times that this isn't a math class.
- Mon Feb 05, 2018 1:04 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Test question [ENDORSED]
- Replies: 16
- Views: 1781
Re: Test question [ENDORSED]
Conservation of energy in the universe.
(I didn't even think of elaborating...)
(I didn't even think of elaborating...)
- Mon Feb 05, 2018 1:02 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: test question 7 [ENDORSED]
- Replies: 6
- Views: 814
Re: test question 7 [ENDORSED]
I don't think we need to know initial deltaH...? What I did was reason that the heat exudes by the ice cube will be the same amount of heat absorbed (so same number just different signs) by the iced tea. That means that the q from phase change of ice (solid--> liquid) + mC(Tfinal - Tinitial) = -mC(T...
- Mon Feb 05, 2018 12:58 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: 8.67c
- Replies: 1
- Views: 275
8.67c
How are we supposed to know what bond equation to use for C6H8? Is it 6C+3H2 or 3C2+3H2 and why? Thanks!
- Sat Jan 20, 2018 8:49 pm
- Forum: Calculating Work of Expansion
- Topic: Battery and reversible process
- Replies: 1
- Views: 438
Battery and reversible process
I'm confused on the thinking point in 8.3: "How could you ensure that an electric battery produced an electric current reversibly?" I don't even understand how a battery works, so I'm pretty stumped.
- Sat Jan 20, 2018 8:09 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: open system
- Replies: 6
- Views: 729
Re: open system
Another way to think of it is to think of a mug of steaming hot chocolate cool down. The hot chocolate/mug lost energy and the surroundings gained energy in form of heat.
- Sat Jan 20, 2018 8:07 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Assigning system/surroundings
- Replies: 5
- Views: 582
Assigning system/surroundings
Will we always be given what is the system/surrounding? And if not, would we be marked incorrect if we defined our system differently?
- Sun Dec 10, 2017 6:27 pm
- Forum: Amphoteric Compounds
- Topic: amphiprotic vs. amphoteric [ENDORSED]
- Replies: 2
- Views: 829
Re: amphiprotic vs. amphoteric [ENDORSED]
An example of what Stephanie said would be water. Water is amphoteric because: Water as a base: H2O + HCl \rightleftharpoons H3O^+ + Cl^- Water as an acid: H2O + NH3 \rightleftharpoons NH4^+ + OH^- In the above reactions, H+ is being donated/accepted. Hence, water is not only amphoteric, but also am...
- Sun Dec 10, 2017 3:14 am
- Forum: Ionic & Covalent Bonds
- Topic: Ca- vs Ca+
- Replies: 2
- Views: 697
Re: Ca- vs Ca+
But why does Ca+ form a shorter and hence stronger bond than Ca-? All I really know about Ca- is that I don't think I've really seen it since it typically exists in its +2 ion state if anything.
- Sat Dec 09, 2017 9:14 pm
- Forum: Lewis Structures
- Topic: Radicals
- Replies: 1
- Views: 262
Radicals
If a structure has a radical, does it have resonance since some bond angles will be different depending on where it's placed? Or does it not matter because resonance only has to do with the bond strength?
- Sat Dec 09, 2017 4:30 pm
- Forum: Bronsted Acids & Bases
- Topic: Amphoteric - BeO
- Replies: 2
- Views: 579
Re: Amphoteric - BeO
Example of BeO acting as an acid would look like: 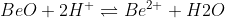
- Fri Nov 24, 2017 10:43 pm
- Forum: Ideal Gases
- Topic: Q and K [ENDORSED]
- Replies: 35
- Views: 3175
Re: Q and K [ENDORSED]
As the others have mentioned, there is no difference in the formula, but it's important to use the right numbers. Use the concentrations/pressures provided when the system isn't at equilibrium to calculate Q.
- Mon Nov 20, 2017 12:00 am
- Forum: Lewis Structures
- Topic: HW Problem 4.13c
- Replies: 2
- Views: 395
Re: HW Problem 4.13c
Adding onto the previous answer, a lewis structure with two double bonded and one single bonded oxygen would allow iodine and two oxygens to have a formal charge of 0 and one oxygen to have a formal charge of -1. This makes sense since oxygen is more electronegative than iodine and will have a sligh...
- Sun Nov 19, 2017 11:48 pm
- Forum: Lewis Structures
- Topic: Organic Lewis Structures
- Replies: 2
- Views: 362
Organic Lewis Structures
I've noticed that a lot of compounds with carbon are written in a different format than I normally expect it to be (ex. HOCO rather than HCO2, CH3CH3 rather than C2H6). When compounds are given in such a fashion, is that always an indicator to the general structure of the compound? Is is a coinciden...
- Sat Nov 11, 2017 1:38 am
- Forum: Lewis Structures
- Topic: Multiple lewis structures for radicals [ENDORSED]
- Replies: 3
- Views: 527
Re: Multiple lewis structures for radicals [ENDORSED]
One useful thing to keep in mind is that hydrogen will almost never have the radical because its valence is already full when it is involved in a single bond. This goes along with the formal charge point that the others above have mentioned.
- Sat Nov 11, 2017 1:35 am
- Forum: Lewis Structures
- Topic: Structure of C2H3N [ENDORSED]
- Replies: 2
- Views: 3501
Re: Structure of C2H3N [ENDORSED]
As for your double bond question, the only time I can think of where you wouldn't want adjacent double bonds is for carbon rings (because that would result in more than an octet for the carbon atoms involved).
- Thu Nov 02, 2017 10:43 am
- Forum: Trends in The Periodic Table
- Topic: Problem 2.61 Part C
- Replies: 2
- Views: 529
Re: Problem 2.61 Part C
Because Na only has to lose one electron before it becomes more stable and has a valence structure with 8 electrons. Na will therefore have a lower ionization energy since it wants to lose that electron. (You can also think of the charges each of the atoms have: Na is +1 while Al is +1 or +3).
- Thu Nov 02, 2017 10:35 am
- Forum: Photoelectric Effect
- Topic: Emission Spectrum
- Replies: 1
- Views: 197
Emission Spectrum
If there are two different elements and in an atom of each, an electron went from a higher orbital to a lower orbital (ex. 4p to 2p), would the emission spectrum be different? My guess is that there would be more lines for the atom with a higher Z, but I'm not really certain.
- Sun Oct 29, 2017 12:38 am
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Precision and constants [ENDORSED]
- Replies: 2
- Views: 364
Re: Precision and constants [ENDORSED]
Seconding Nicole's answer. Using all the figures that are provided for constants in the sheet ensures that you won't have rounding errors for your final answer.
- Sun Oct 29, 2017 12:35 am
- Forum: Significant Figures
- Topic: no Sig Figs in question [ENDORSED]
- Replies: 4
- Views: 856
Re: no Sig Figs in question [ENDORSED]
Does that mean that we have to report 6 sig figs in our final answer if we use Planck's constant since the provided constants sheet provides 6 sig figs? I'm not sure if my weak handheld calculator stores enough figures for that.
- Sun Oct 29, 2017 12:32 am
- Forum: Trends in The Periodic Table
- Topic: Electron affinity
- Replies: 1
- Views: 213
Electron affinity
I still don't really understand why energy is released when an electron is added to a gas phase atom. Is it because the atom becomes more stable?
- Sun Oct 22, 2017 10:59 pm
- Forum: Photoelectric Effect
- Topic: Exam Question Clarification [ENDORSED]
- Replies: 3
- Views: 342
Re: Exam Question Clarification [ENDORSED]
I agree with Miranda-- I think that increasing intensity meant increasing the number of photons, not the frequency since it was a concept question looking to test whether students recognized the significance of the photoelectric experiments.
- Sun Oct 22, 2017 10:57 pm
- Forum: Properties of Light
- Topic: Velocity [ENDORSED]
- Replies: 13
- Views: 1625
Re: Velocity [ENDORSED]
I'm still confused. Can we use the speed of light as our velocity element when the direction part of the vector component is negligible in our calculations?
- Sun Oct 22, 2017 10:55 pm
- Forum: Ionic & Covalent Bonds
- Topic: Chemical formula?
- Replies: 5
- Views: 711
Re: Chemical formula?
Hi! In addition to what others have mentioned above, it's helpful to know the charges of the common elements on the table. For example, Group I all has a +1 charge, Group II has +2, etc. Once you know the charges of the elements in your compound, you can find out how many atoms of an element are in ...
- Thu Oct 12, 2017 1:27 am
- Forum: Properties of Light
- Topic: HW Problem 1.3
- Replies: 4
- Views: 610
Re: HW Problem 1.3
I think that the relationship between frequency and the extent of the change in the electrical field can be explained by the equation
E(upper)-E(lower) = h*v
In the equation, frequency and E(upper)-E(lower) are on opposite sides of the equal sign and are proportionally related.
E(upper)-E(lower) = h*v
In the equation, frequency and E(upper)-E(lower) are on opposite sides of the equal sign and are proportionally related.
- Thu Oct 12, 2017 1:23 am
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Visualizing Heisenberg Uncertainty Equation
- Replies: 1
- Views: 322
Visualizing Heisenberg Uncertainty Equation
While I understand while reading that the position and momentum of a small object cannot be known simultaneously, I have a hard time visualizing this because the concept is so foreign in the sense that the objects we interact with daily are large enough to determine both position and momentum. Is th...
- Fri Oct 06, 2017 9:47 am
- Forum: SI Units, Unit Conversions
- Topic: Significant Figures [ENDORSED]
- Replies: 9
- Views: 2111
Re: Significant Figures [ENDORSED]
When you read the problem, there is usually a given mass/length/time. Take part b of M7 for example: What mass of boron can be produced when 125kg of boron oxide is heated with 125kg of magnesium? We are given 125kg, which has 3 sig figs. Hence, our answer will be reported in 3 sig figs. This isn't ...
- Wed Oct 04, 2017 10:15 pm
- Forum: SI Units, Unit Conversions
- Topic: Limiting Reagents [ENDORSED]
- Replies: 8
- Views: 1088
Re: Limiting Reagents [ENDORSED]
For M9 (and other net ionic problems), first write the complete chemical equation. Then rewrite the chemical equation, except this time writing down ions that separate (ex. salts like NaCl, strong acids like HCl). Cancel out any ions that are on both sides. I don't think we have to know this for the...
- Wed Oct 04, 2017 10:11 pm
- Forum: SI Units, Unit Conversions
- Topic: Sig Figs
- Replies: 12
- Views: 3314
Re: Sig Figs
I find that a useful tip for finding the number of sig figs is to put the number into scientific notation! So for 460, it'd be 4.6 x 10^2. In scientific notation, it's a lot easier to check sig figs.

