Search found 31 matches
- Sun Mar 18, 2018 8:17 pm
- Forum: Calculating Work of Expansion
- Topic: Final Question W18: Reversible vs. Irreversible External Pressure
- Replies: 4
- Views: 597
Re: Final Question W18: Reversible vs. Irreversible External Pressure
Also, the work for a reversible expansion is greater than the work for an irreversible expansion.
- Wed Mar 14, 2018 6:42 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: pH
- Replies: 1
- Views: 317
Re: pH
Idk why this is in 14b, but pH is the -log[H+], so if we take the 10^-pH, we get the [H+]
- Wed Mar 14, 2018 5:44 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Isothermal Changes
- Replies: 2
- Views: 408
Re: Isothermal Changes
Remember heat does not equal temperature. Isothermal means temperature is held constant, but heat is not restricted. As a result, deltaU equals 0. When we plug this into the first law of thermodynamics equation of deltaU=q+w, we get q=-w.
- Wed Mar 14, 2018 5:38 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Slow Step
- Replies: 4
- Views: 514
Re: Slow Step
In mechanisms, everything that comes after the slow-step is not used when determining the overall rate law of the proposed mechanism. It's only when there's a step before the slow, rate-determining step that we may use the pre-equilibrium approach.
- Fri Mar 09, 2018 11:26 pm
- Forum: General Rate Laws
- Topic: Pseudo-Reactions [ENDORSED]
- Replies: 2
- Views: 581
Re: Pseudo-Reactions [ENDORSED]
The Pseudo-rate law is used whenever there is a reaction in which there are multiple reactants affecting the rate of the reaction. For example, if rate=k[A]^N[B]^M[C]^L, then we would need to use the pseudo-rate law in order to calculate the orders of the individual reactants. We do this by saying t...
- Tue Mar 06, 2018 8:34 pm
- Forum: General Rate Laws
- Topic: Units in 15.17 vs. 15.19
- Replies: 2
- Views: 398
Units in 15.17 vs. 15.19
How do we know when we should switch our units from mmol to mol? For example, in question 15.17, the solutions manual keeps the concentrations of A and B as well as the rate in terms of mmol, but in question 15.19, the concentrations of A, B, and C as well as the rate are converted into mol. Is ther...
- Tue Feb 27, 2018 10:06 am
- Forum: General Rate Laws
- Topic: Negative Orders
- Replies: 1
- Views: 234
Negative Orders
In the textbook, there is an example of the Rate law of the decomposition of ozone, Rate=k [O3]^2[O2]^-1. It says that some species, typically products, do not have positive orders, meaning an increase in concentration correlates to a decrease in rate. Are we going to be tested on this concept?
- Mon Feb 26, 2018 9:13 pm
- Forum: General Rate Laws
- Topic: Examples
- Replies: 3
- Views: 563
Re: Examples
Unless experimental data is given to you with which you can find the reaction order, the reaction order will be given to you.
- Mon Feb 26, 2018 9:12 pm
- Forum: First Order Reactions
- Topic: Ways of determining what order reactions are
- Replies: 2
- Views: 423
Re: Ways of determining what order reactions are
The order of a reaction is found by adding up the orders of all the reactants. To find the order of a reactant, we can use experimental data given to us. If an increase in concentration of a reactant doesn't change the reaction rate, then that reactant is a 0 ordered reactant. If the ratio of concen...
- Wed Feb 21, 2018 3:48 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Nernst equation usage
- Replies: 3
- Views: 627
Re: Nernst equation usage
The Nernst equation is used when finding the E under non-standard conditions. There are multiple versions of the equation, but they all mean the same thing and can be used interchangeably. I believe the equation you are talking about is E°=(RTlnK)/nF, which is pretty much the same thing again. This ...
- Wed Feb 21, 2018 3:44 pm
- Forum: Balancing Redox Reactions
- Topic: Writing Half-Reactions
- Replies: 6
- Views: 917
Re: Writing Half-Reactions
The half-reactions given in the appendix of the textbook give the reduction reaction. Since the anode is where oxidation takes place, we would need to flip the half-reaction given in order to get the oxidation half-reaction. The cathode is where reduction takes place, so no such flipping is needed.
- Wed Feb 21, 2018 3:41 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Q & K?
- Replies: 4
- Views: 612
Re: Q & K?
If Q>K then that means there is more product than at the equilibrium concentration. As such, LeChatelier's principle tells us that the reactants will be favored. If Q<K then that means there is more reactant than at the equilibrium concentration, so the products will be favored.
- Wed Feb 21, 2018 3:39 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Homework Question 14.13 Part (d)
- Replies: 1
- Views: 749
Re: Homework Question 14.13 Part (d)
We use Au(s) for the anode because it needs a solid conducting metal for the electrode. This question is an example of a concentration cell, where both the anode and cathode are the same conducting metal.
- Fri Feb 16, 2018 10:00 am
- Forum: Balancing Redox Reactions
- Topic: 14.5 c
- Replies: 2
- Views: 390
Re: 14.5 c
The reducing agent is the one that gives away its electrons, causing the other molecule to be reduced, while the oxidizing agent is the one that receives electrons from the other molecule, causing the other molecule to be oxidized. As a result, the molecule getting oxidized is the reducing agent whi...
- Fri Feb 16, 2018 9:55 am
- Forum: Balancing Redox Reactions
- Topic: Cl2 and Cl -
- Replies: 2
- Views: 1388
Re: Cl2 and Cl -
I'm not exactly sure what you're asking but in Cl-, the charge is -1. However, in Cl2 gas, the Cl's are neutral, 0 charge. As a result, we get the half reaction Cl2 + 2e- --> 2Cl-. We have 2 Cl- ions because Cl2 gas has 2 Cls and we need to balance both sides of the reaction
- Fri Feb 16, 2018 9:52 am
- Forum: Balancing Redox Reactions
- Topic: The use of H3O+ versus H+
- Replies: 2
- Views: 895
Re: The use of H3O+ versus H+
We would use H+ in these situations
- Fri Feb 09, 2018 10:16 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode and cathode
- Replies: 9
- Views: 1439
Re: Anode and cathode
The anode is the one that oxidizes and is usually on the left and cathode is the one that reduces and is usually on the right
- Fri Feb 09, 2018 10:14 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: delta G=0
- Replies: 6
- Views: 1540
Re: delta G=0
Delta G=delta H - TdeltaS so if delta G=0 then either both delta H and delta S are zero or delta H equals T times delta S
- Fri Feb 09, 2018 10:12 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Entropy
- Replies: 7
- Views: 724
Re: Entropy
S^o r is the standard reaction entropy
- Fri Feb 02, 2018 10:33 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Free Energy and Pressure
- Replies: 4
- Views: 482
Re: Free Energy and Pressure
Enthalpy is heat under constant pressure, so wouldn't a change in pressure mean pressure isn't being held constant?
- Fri Feb 02, 2018 10:28 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: 9.7
- Replies: 7
- Views: 736
Re: 9.7
Also, the value of Cp as well as Cv will be given to us on exams.
- Fri Feb 02, 2018 10:18 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Micro states
- Replies: 5
- Views: 651
Re: Micro states
Microstates are the positions molecules can take and are what we can use to determine entropy.
- Thu Jan 25, 2018 7:24 pm
- Forum: Calculating Work of Expansion
- Topic: Homework 8.11
- Replies: 6
- Views: 748
Re: Homework 8.11
We get the equation w=-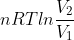 from the ideal gas law PV=nRT. We substitute P=(nRT)/V into the integral w=
from the ideal gas law PV=nRT. We substitute P=(nRT)/V into the integral w=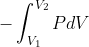 and get w=
and get w=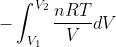 which gives us w=-nRT(lnV2-lnV1), which is rearranged as w=
which gives us w=-nRT(lnV2-lnV1), which is rearranged as w=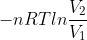 .
.
- Thu Jan 25, 2018 7:12 pm
- Forum: Calculating Work of Expansion
- Topic: Irreversible Expansion
- Replies: 3
- Views: 577
Re: Irreversible Expansion
Dr. Lavelle gave the example in lecture of a piston with internal pressure of 2 atm and an external pressure of 1 atm. With nothing being done to keep the piston in place (such as a pin being inserted), the internal pressure would push up against the weaker external pressure in a sudden movement. Th...
- Thu Jan 25, 2018 7:04 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bond enthalpies vs enthalpy of rxn - conceptual [ENDORSED]
- Replies: 3
- Views: 660
Re: Bond enthalpies vs enthalpy of rxn - conceptual [ENDORSED]
No, breaking bonds does not release energy, it requires energy to break. As a result, breaking a bond is an endothermic process.
- Thu Jan 18, 2018 9:04 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Delta U?
- Replies: 7
- Views: 10659
Re: Delta U?
Also remember 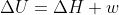 when under constant pressure
when under constant pressure
- Thu Jan 18, 2018 9:00 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Why is using bond enthalpies the least accurate method of finding reaction enthalpies?
- Replies: 5
- Views: 10165
Re: Why is using bond enthalpies the least accurate method of finding reaction enthalpies?
Only diatomic molecules have accurate bond enthalpy measurements, all other molecules' bond enthalpies are taken as averages
- Thu Jan 18, 2018 8:48 pm
- Forum: Phase Changes & Related Calculations
- Topic: 8.37
- Replies: 3
- Views: 477
Re: 8.37
The enthalpy of vaporization is given in the unit kJ/mol. In part (a), we are given the total heat required to vaporize the methane as well as the moles of methane present. In order to find the enthalpy of vaporization, all we do is divide the kJ's of heat required by the 0.579 mol methane to get th...
- Wed Jan 10, 2018 8:19 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Is bond enthalpy the same as dissociation energy? [ENDORSED]
- Replies: 2
- Views: 241
Re: Is bond enthalpy the same as dissociation energy? [ENDORSED]
Bond enthalpy is not strictly the energy required to break the bond, it's also equivalent to the energy released when the same bond is formed. So remember that bond enthalpy is positive for the reactants (since breaking bonds requires energy) and that bond enthalpy is negative for the reactants (for...
- Wed Jan 10, 2018 8:15 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Equations
- Replies: 6
- Views: 585
Re: Equations
Enthalpy of sublimation = H of vapor - H of solid
- Wed Jan 10, 2018 8:00 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Specific heat of water
- Replies: 3
- Views: 349
Re: Specific heat of water
The liquid form of water has a greater heat capacity than both the solid and gaseous phases because in the liquid phase, the network of hydrogen bonds is allowed to be free, and these intermolecular bonds cause liquid water to need more energy(heat) to be broken.

