What is the chemical formula for the molecules in candy?
Carbon-Holmium-Cobalt-Lanthanum-Tellurium
or CHoCoLaTe
Search found 55 matches
- Sun Mar 18, 2018 6:06 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3624962
- Sun Mar 18, 2018 5:52 pm
- Forum: Calculating Work of Expansion
- Topic: Final Question W18: Reversible vs. Irreversible External Pressure
- Replies: 4
- Views: 594
Re: Final Question W18: Reversible vs. Irreversible External Pressure
In irreversible expansion, external pressure is held constant, while for reversible expansion, external pressure is changing. You can tell by the equations that are used under each condition as irreversible expansion uses -P(deltaV) and reversible expansion uses -nRTln(V2/V1).
- Sun Mar 18, 2018 5:43 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: State Functions
- Replies: 4
- Views: 762
State Functions
How many state functions should we have gotten as an answer for the question on the final?
- Sat Mar 10, 2018 4:10 pm
- Forum: Zero Order Reactions
- Topic: Zero Order and Catalysts [ENDORSED]
- Replies: 2
- Views: 2872
Zero Order and Catalysts [ENDORSED]
What is connection between a zero order reaction and the presence of a catalyst?
- Sat Mar 10, 2018 4:06 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Substitution Reactions [ENDORSED]
- Replies: 1
- Views: 269
Substitution Reactions [ENDORSED]
On Friday, Lavelle explained a bit about substitution reactions and nucleophiles. Do we need to understand this concept for the final? If so can someone explain how these reactions connect to reaction profiles that are based on Gibbs Free Energy rather than Activation energy?
- Sat Mar 10, 2018 12:34 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Methods to approach proposed reaction mechanisms
- Replies: 1
- Views: 277
Methods to approach proposed reaction mechanisms
Can someone explain the difference between the steady-state and the pre-equilibrium approach?
- Sun Mar 04, 2018 9:11 pm
- Forum: First Order Reactions
- Topic: Order Reactions
- Replies: 3
- Views: 485
Order Reactions
Will we need to derive each of the different order reactions from the differential rate law for any future tests?
- Wed Feb 28, 2018 12:51 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Rate constant
- Replies: 2
- Views: 472
Rate constant
What is the difference between a rate constant and an equilibrium constant?
- Tue Feb 27, 2018 11:48 pm
- Forum: Second Order Reactions
- Topic: 15.35
- Replies: 1
- Views: 252
15.35
For this question, we are meant to calculate the time needed for a concentration to decrease to a) 1/16th b) 1/4th c) 1/5th the original value. We are given the half-life of the second-order reaction and the initial concentration. In the solution manual we use the second-order half life equation to ...
- Tue Feb 27, 2018 12:29 am
- Forum: First Order Reactions
- Topic: First Order Reaction Equations
- Replies: 3
- Views: 554
First Order Reaction Equations
How do you know when to use 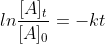
or when to use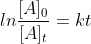 ?
?
Are there specific instances when we use each?
or when to use
Are there specific instances when we use each?
- Mon Feb 26, 2018 1:08 am
- Forum: First Order Reactions
- Topic: Direct Proportions and First Order
- Replies: 5
- Views: 651
Direct Proportions and First Order
Why does a directly proportional relationship between the rate of the reaction and the concentration of the reactants indicate a first order?
- Mon Feb 26, 2018 12:38 am
- Forum: General Rate Laws
- Topic: Unique Rate of Reactions
- Replies: 3
- Views: 486
Unique Rate of Reactions
What is the unique rate of reactions? How would you calculate it?
- Mon Feb 26, 2018 12:37 am
- Forum: General Rate Laws
- Topic: rate of reaction vs rate law
- Replies: 2
- Views: 441
Re: rate of reaction vs rate law
The rate of the reaction can be found as the change in concentration of product or reactant over the change in time. The rate law (aka differential rate law) is found using the equation k[R]^n in which n is the order of the reactant, [R] represents the concentration of the reactant, and k is the rat...
- Mon Feb 19, 2018 12:31 am
- Forum: Balancing Redox Reactions
- Topic: Standard Reduction Potential
- Replies: 3
- Views: 478
Standard Reduction Potential
How come the standard reduction potentials stay the same when half reactions are balanced?
- Sun Feb 18, 2018 7:17 pm
- Forum: Balancing Redox Reactions
- Topic: Balancing
- Replies: 3
- Views: 405
Re: Balancing
I believe you can determine how many electrons to add to a half reaction based on the change in oxidation number of the element being reduced or oxidized. The number of electrons added would then make up for the difference to make both sides of the reaction balanced
- Sun Feb 18, 2018 6:56 pm
- Forum: Balancing Redox Reactions
- Topic: Half Reaction
- Replies: 3
- Views: 453
Half Reaction
When giving a full redox reaction and asked to write either the oxidation or reduction half-reactions, can we use the elements alone with an oxidation number for each half reaction? or are we expected to utilize the full compound when writing the half reaction? For example, in 14.1 we are asked to w...
- Sat Feb 10, 2018 9:31 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Molar entropy v standard entropy of formation
- Replies: 3
- Views: 2880
Molar entropy v standard entropy of formation
What is the difference between molar entropies and standard entropy of formation? Why do we use molar entropies to find the standard reaction entropies and not standard entropies of formation?
- Sat Feb 10, 2018 9:14 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Stability
- Replies: 2
- Views: 370
Stability
Why can we look at the Gibbs Free Energies of Formation to determine stability? Why would a positive free energy of formation indicate an unstable compound while negative ones indicate stable compounds?
- Sat Feb 10, 2018 8:16 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Calculating Gibbs Free Energy [ENDORSED]
- Replies: 2
- Views: 478
Calculating Gibbs Free Energy [ENDORSED]
When asked to calculate Gibbs Free Energy when should you use the equation deltaH - T*deltaS compared to using the standard formation values and subtract the sum of the products from the sum of the reactants?
- Sun Feb 04, 2018 11:05 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: 9.31
- Replies: 2
- Views: 386
9.31
For future exams will we be required to know the states of substances like in 9.31 in which we needed to know the states of ethene and polyethylene to determine which has the higher molar entropy?
- Sun Feb 04, 2018 10:35 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Higher Molar Entropy
- Replies: 4
- Views: 873
Higher Molar Entropy
How would you define molar entropy? What are some good ways to determine if a substance or compound has a higher or lower molar entropy?
- Sun Feb 04, 2018 10:19 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 9.19 Finding standard entropy of water
- Replies: 1
- Views: 271
9.19 Finding standard entropy of water
Can someone explain further why when determining the standard entropy of water at 85 degrees C you need to find the entropy change when heating the reactants to boiling point and the change when cooling the products back down to 85 and then finally add those changes with the standard entropy at 100 ...
- Sun Jan 28, 2018 9:41 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Deriving the equation for changes in pressure
- Replies: 1
- Views: 232
Deriving the equation for changes in pressure
How would you derive the equation you would use for entropy changes due to changes in pressure?
- Sun Jan 28, 2018 9:28 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Isothermal Reversible
- Replies: 3
- Views: 837
Isothermal Reversible
When a problem mentions that an entropy change occurred isothermally do we assume that the process was also reversible? Why or why not?
- Sun Jan 28, 2018 9:03 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Calculating Entropy Change [ENDORSED]
- Replies: 2
- Views: 199
Calculating Entropy Change [ENDORSED]
When using the Second Law of Thermodynamics, how do we know when q will be negative? Does it depends on whether q is referring to the system or surroundings? or when q is referring to heat lost or gain?
- Thu Jan 18, 2018 2:42 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Homework 8.15
- Replies: 3
- Views: 441
Homework 8.15
Why is part c, q<0, always false in adiabatic processes, in which no energy is transferred as heat? Is this because in these processes, q is simply 0 and would not be negative for the situation? What is an example of a situation in which q would be negative?
- Thu Jan 18, 2018 1:54 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Test
- Replies: 1
- Views: 210
Test
For our test would we be provided with the standard enthalpies of formation, if given a question asking to calculate the standard reaction enthalpy for a reaction?
- Thu Jan 18, 2018 1:51 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Extensive v Intensive Properties
- Replies: 2
- Views: 332
Extensive v Intensive Properties
What is the difference between an extensive and an intensive property, and how do they relate to heat capacity?
- Thu Jan 18, 2018 1:48 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Internal Energy
- Replies: 4
- Views: 539
Internal Energy
What is the difference between internal energy and enthalpy?
- Sun Jan 14, 2018 8:47 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Molar Heat Capacity
- Replies: 4
- Views: 190
Re: Molar Heat Capacity
Yes as the molecule becomes more complex the heat capacity will increase as a result. Homework problem #29 in the textbook deals with this concept as NO2 is seen to have a higher molar heat capacity compared to NO due to the additional oxygen allowing for a greater absorption of added energy.
- Sat Jan 13, 2018 11:35 pm
- Forum: Phase Changes & Related Calculations
- Topic: Steam Burning
- Replies: 7
- Views: 948
Steam Burning
Can someone give again the explanation Lavelle shared last Monday about why steam burns a lot more and how that relates to phase changes?
- Thu Jan 11, 2018 12:19 pm
- Forum: Calculating Work of Expansion
- Topic: Work conversion [ENDORSED]
- Replies: 1
- Views: 287
Work conversion [ENDORSED]
Do we need to know the conversion of liter-atmospheres to joules?
- Sat Dec 09, 2017 8:17 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Adding a compound to reaction
- Replies: 2
- Views: 2089
Re: Adding a compound to reaction
If a solid is added, it should have not effect since it is not included in the equilibrium constant. May I ask which specific problem you are referring to in his practice test?
- Sat Dec 09, 2017 8:13 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: X in ICE table
- Replies: 3
- Views: 3618
Re: X in ICE table
One way to determine is through what the problem gives you. If only the amount of the initial reactant is given, then the initial concentration of the products would be 0. This then means that the reactants will be consumed to make products until the reaction reaches equilibrium. Since the reactants...
- Sat Dec 09, 2017 3:33 pm
- Forum: Lewis Structures
- Topic: NO2 Lewis Structure
- Replies: 2
- Views: 2790
Re: NO2 Lewis Structure
NO2 cannot be drawn with two double bonds and one lone pair on the N central atom because it violates the octet rule. N cannot have exceed the octet rule because it does not have empty d orbitals unlike many of the third period elements, which uses their empty d orbitals to accommodate more electrons.
- Sat Dec 09, 2017 3:27 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Changing Kc
- Replies: 2
- Views: 4972
Re: Changing Kc
The value of Kc can change when stoichiometric coefficients are halved because when calculating Kc itself it requires the stoichiometric coefficients used in the chemical equation as exponents of the reactants or products. To demonstrate using an equation that had A + 2B = 4C and assuming they are a...
- Sat Dec 09, 2017 11:29 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sits vs shifts left/right
- Replies: 1
- Views: 466
Re: Sits vs shifts left/right
I believe the term "sits to the left" means there are more reactants at equilibrium, which can be seen through determining if K is small (K<10^-3). The term "sits to the right" then refers to a larger amount of products, which can be seen through determining that K is large (K> 1...
- Sat Dec 09, 2017 4:05 am
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: 12.39
- Replies: 1
- Views: 299
12.39
Can someone explain how to find the pKa of NH3OH and (CH3)2NH2? I understand the need to subtract the pKb from 14 to find the pKa, but how do you find the pKb in the first place?
- Fri Dec 08, 2017 11:15 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Conductivity of Acids and Bases [ENDORSED]
- Replies: 1
- Views: 469
Conductivity of Acids and Bases [ENDORSED]
Do we need to know the conductivity of certain acids and bases for the final?
- Fri Dec 08, 2017 10:54 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: XeO2F2
- Replies: 3
- Views: 4821
Re: XeO2F2
XeO2F2 is polar and it helps to identify this through drawing out the lewis structure. In doing so, you can see that Xe has 5 electron densities with 4 bonded pairs and one lone pair. The single lone pair gives the compound a seesaw shape which then would be polar
- Fri Dec 08, 2017 8:19 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Polarity
- Replies: 1
- Views: 234
Re: Polarity
The molecular shape can be used to determine polarity through observing the lone pairs and the resulting shape of the compound. If there are no lone pairs the compound should be nonpolar, but once there are lone pairs it is important to observe the shape to see if any dipole moments exists and if th...
- Fri Dec 08, 2017 8:12 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: #45 in Lyndon's Practice Exam
- Replies: 1
- Views: 216
#45 in Lyndon's Practice Exam
Can someone explain again the concept behind why the fact that electron withdrawing atoms can stabilize anions by delocalizing the negative charge is true?
- Fri Dec 08, 2017 8:08 pm
- Forum: Biological Examples
- Topic: Biological Importance
- Replies: 2
- Views: 455
Biological Importance
What are some important things we need to know regarding the biological aspects/importance of coordination complexes? For example, we need to know 4 myoglobins form hemoglobins.
- Mon Nov 27, 2017 5:12 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Writing reaction quotient or equilibrium expression
- Replies: 5
- Views: 482
Writing reaction quotient or equilibrium expression
For writing the reaction quotient, Q, or equilibrium expression, K, when do we write it as partial pressure with the capital P then the molecule as a subscript in contrast to as a concentration with the molecule surrounded in brackets? For example in 11.3, it asks to write the equilibrium expression...
- Tue Nov 21, 2017 10:54 pm
- Forum: Hybridization
- Topic: 4.95 composition of the pi bond
- Replies: 2
- Views: 387
4.95 composition of the pi bond
Can someone elaborate why the pi bonds for the structure in 4.95 have only the 2p hybridization in contrast to the sigma bonds with the sp2 hybridization and perhaps how you would come to this conclusion through observing the lewis structure?
- Tue Nov 21, 2017 2:35 pm
- Forum: Naming
- Topic: Ligand Names
- Replies: 6
- Views: 667
Ligand Names
There is a table in the book that includes a list of common ligands and their corresponding formulas. Are we required to know all of these for the test or final?
- Tue Nov 21, 2017 2:31 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: CH2CL2 4.25
- Replies: 2
- Views: 197
CH2CL2 4.25
In the problem 4.25, why is CH2CL2 polar? I understand the Cl is more electronegative and thus there is a stronger pull of the electrons/negative charge towards the Cl. However, when drawing the lewis structure couldn't the Cl be draw opposite to each other to make these charges cancel?
- Sat Nov 18, 2017 5:33 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lewis Structures and VSEPR Shape
- Replies: 4
- Views: 600
Lewis Structures and VSEPR Shape
When drawing lewis structures, do we need to draw them in a way that reflect the molecular shape? For example in sulfur tetrafluoride (SF4), during lecture we drew it with the lone pair on the left side of the sulfur (the equatorial plane). I believe this reflects the most stable shape with the leas...
- Wed Nov 08, 2017 11:46 pm
- Forum: Lewis Structures
- Topic: Lewis Structure [ENDORSED]
- Replies: 4
- Views: 565
Lewis Structure [ENDORSED]
When asked to find the lewis structure, do you automatically write the structure with the lowest energy?
- Sun Nov 05, 2017 7:35 pm
- Forum: Lewis Structures
- Topic: 3.39
- Replies: 1
- Views: 221
3.39
Would someone be able to explain why the lewis structures for the compounds are written separately and not with any connecting bonds? For example in part a, the chlorine anion is not bonded to the NH3 and stands alone with 4 lone pairs. How would you also be able to recognize that you need do this f...
- Wed Nov 01, 2017 7:37 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Multiple Electron Atoms
- Replies: 2
- Views: 434
Multiple Electron Atoms
Can someone elaborate further on the effective nuclear charge and how it relates to electron shielding in multiple electron atoms?
- Sun Oct 29, 2017 10:28 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Speed of light [ENDORSED]
- Replies: 1
- Views: 256
Re: Speed of light [ENDORSED]
Both values are correct. However, the specific value you would use for a problem is all dependent on the number of significant figures required for the problem. For example if only 2 sig figs are required, 3.0* 10^8 m*s^-1 would be used in the problem.
- Fri Oct 20, 2017 10:51 pm
- Forum: SI Units, Unit Conversions
- Topic: Quantum Mechanics
- Replies: 4
- Views: 797
Re: Quantum Mechanics
I believe to determine which has a higher electron affinity, you would need to refer to the periodic table. Electron affinities tend to be the highest towards the right of the periodic table. You would apply this electron affinity trend by comparing where each element is locating in relation to each...
- Fri Oct 13, 2017 4:49 pm
- Forum: Photoelectric Effect
- Topic: Lyman Series v Balmer series
- Replies: 2
- Views: 396
Re: Lyman Series v Balmer series
The main difference between the Lyman and Balmer series is the energy level in which electrons come to rest. For the Lyman series n=1, while for the Balmer series n=2. Another important characteristic to note is that the Lyman series is found within the ultraviolet spectrum, while the Balmer series ...
- Fri Oct 06, 2017 9:36 am
- Forum: Balancing Chemical Reactions
- Topic: L35
- Replies: 2
- Views: 649
Re: L35
Before doing anything it is important to check that all the equations are balanced! 1. Convert the 2.50 t to grams. This is best done by converting first to kilograms and then converting that value to grams. 2. Convert the g of NaBr to moles by dividing by the molar mass of NaBr. 3. Once you found t...

