Search found 51 matches
- Thu Mar 15, 2018 5:03 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: 8.49
- Replies: 2
- Views: 436
Re: 8.49
I think that we assume that this is an ideal gas because we assume that it only interacts with the water vapor and is not affected by other external forces.
- Thu Mar 15, 2018 4:58 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Collision theory for Reaction Mechanisms
- Replies: 2
- Views: 465
Re: Collision theory for Reaction Mechanisms
Hi if I recall correctly, Dr. Lavelle gave the example of the throwing tennis balls in the air. The probability of throwing three balls in the air and having them collide at the exact same time is lower than the probability of throwing two balls in the air and having them collide at the exact same t...
- Thu Mar 15, 2018 4:38 pm
- Forum: *Amines
- Topic: Memorization
- Replies: 2
- Views: 1351
Re: Memorization
Here are the connections that I made for each functional group Alcohol --> Ether (The H in OH becomes an R group) Alcohol --> Aldehyde (Dehydrogenation OH becomes H + O) Aldehyde --> Ketone (H becomes R group = reason why Ketone is in the middle of the carbon chain unlike Aldehydes which are found a...
- Sat Mar 10, 2018 5:45 pm
- Forum: Second Order Reactions
- Topic: 15.35
- Replies: 6
- Views: 1124
Re: 15.35
We cannot simply multiply the half life by a factor of 4 because the half life of a second order reaction is reliant on the initial concentration of the reactant (while the first order half life reaction only relies on the equilibrium constant.) Second order half life equation: 1/k[A] o First order ...
- Sat Mar 10, 2018 5:38 pm
- Forum: Experimental Details
- Topic: Homework Problem 15.3 Part C
- Replies: 5
- Views: 1164
Re: Homework Problem 15.3 Part C
Note that the unique rate is the rate of formation, and the rate of formation for reactants is negative (because reactants are consumed in the reaction). Therefore, we must put a negative sign in front of the reactant's rate of formation to give it a positive value that equals the positive rate of f...
- Sat Mar 10, 2018 5:33 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Negative Order
- Replies: 7
- Views: 15915
Re: Negative Order
A negative first order would occur if a the rate is halved when the concentration of a reactant is doubled.
- Sat Mar 03, 2018 9:27 pm
- Forum: General Rate Laws
- Topic: half-life
- Replies: 8
- Views: 1010
Re: half-life
Half life can also tell you how fast a substance decays.
ie. A substance that has a half life of 10 seconds has a faster rate of decay in comparison to a substance that has a half life of 10 days.
ie. A substance that has a half life of 10 seconds has a faster rate of decay in comparison to a substance that has a half life of 10 days.
- Sat Mar 03, 2018 9:22 pm
- Forum: General Rate Laws
- Topic: Test 3 - Derivations
- Replies: 4
- Views: 710
Re: Test 3 - Derivations
Although the formulas for Rate Laws and Integrated Rate Laws are given, it is helpful to know how to derive the integrated rate law to have a better understanding of the concepts.
- Sat Mar 03, 2018 2:46 pm
- Forum: First Order Reactions
- Topic: Naming Orders
- Replies: 3
- Views: 588
Naming Orders
I know that the overall reaction order for:
RATE= k[NH4+]n [NO2-]m
is n+m (if n=1 and m=1) the overall reaction rate is 2
But what do we call the individual orders (only n or only m)?
RATE= k[NH4+]n [NO2-]m
is n+m (if n=1 and m=1) the overall reaction rate is 2
But what do we call the individual orders (only n or only m)?
- Fri Feb 23, 2018 7:49 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Solving for Q when given molarity and partial pressure
- Replies: 3
- Views: 2164
Solving for Q when given molarity and partial pressure
How do we solve for Q when we are given both molarity and partial pressure?
What if the partial pressures in 14.37a were different?
What if the partial pressures in 14.37a were different?
- Fri Feb 23, 2018 7:39 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Inert Electrode
- Replies: 4
- Views: 1463
Re: Inert Electrode
Without an inert solid metal conductor, a cell with only aqueous ions would not be able to transfer electrons from the anode to the cathode.
- Fri Feb 23, 2018 7:34 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Finding n
- Replies: 15
- Views: 1962
Re: Finding n
An example might be: if the oxidation half reaction produces 2 mol of e- and reduction half reaction produces 3 mol of e- you’d have to multiply the oxidation half rxn by a factor of 3 and the reduction half rxn by a factor of 2 because the moles of e- must cancel in this case 2mol e- * 3 = 6mol e- ...
- Sat Feb 17, 2018 8:38 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Adiabatic Reaction
- Replies: 2
- Views: 440
Re: Adiabatic Reaction
Additionally, if you were asked when does Delta U = q, your answer would be:
When w=0 because Delta U=w and q=0 under adiabatic conditions
When w=0 because Delta U=w and q=0 under adiabatic conditions
- Sat Feb 17, 2018 8:34 pm
- Forum: Balancing Redox Reactions
- Topic: Cl2 and Cl -
- Replies: 2
- Views: 1409
Re: Cl2 and Cl -
If you are wondering why Cl2 has an oxidation number of 0, it is because neutral uncombined atoms or atoms in pure element is assigned an oxidation number of 0.
ex. Cl2(g), Na(s), Hg(l)
ex. Cl2(g), Na(s), Hg(l)
- Sat Feb 17, 2018 8:20 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidizing & Reducing Agents
- Replies: 6
- Views: 1234
Re: Oxidizing & Reducing Agents
In the image below, the Reducing Agent (A) is oxidized because it loses its electrons. The Oxidizing Agent (B) is reduced because it gains electrons.
- Fri Feb 09, 2018 9:36 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 9.43 Using Cp vs Cv?
- Replies: 2
- Views: 433
Re: 9.43 Using Cp vs Cv?
You would only use the Cv,m and Cp,m values (3/2R, 5/2R, etc) as the heat capacity for ideal gases. Additionally, if water were a vapor, Cp,m would equal 4R not 5/2R because water is a nonlinear molecule.
- Fri Feb 09, 2018 9:18 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 9.57 [ENDORSED]
- Replies: 1
- Views: 261
9.57 [ENDORSED]
Can someone explain why the units for the answers are "kJ/mol"? Since we are decomposing 2 moles of Hydrogen Peroxide, shouldn't the answer be in just "kJ" because we already accounted for the 2 moles of Hydrogen Peroxide? In the Solutions Manual: for part A, delta G is -233.6 kJ...
- Mon Feb 05, 2018 12:24 am
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: 9.27 hypothetical (d)
- Replies: 1
- Views: 271
9.27 hypothetical (d)
If we were given 1mol Ar(g) @ 1atm and .5mol Ar(g) (instead of 1mol) @ 2atm would the entropy be the same or would 1mol @1 atm still have more entropy because of its larger volume?
- Fri Feb 02, 2018 9:13 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Assume ideal gas?
- Replies: 4
- Views: 598
Re: Assume ideal gas?
Unless we learn how to calculate the entropy changes for non-ideal gases, I believe that --for this class-- we assume that all gases are ideal gases.
- Thu Feb 01, 2018 4:11 pm
- Forum: Calculating Work of Expansion
- Topic: Expansion
- Replies: 3
- Views: 458
Re: Expansion
In the internal energy equation
Delta U = q+w
our w value would be negative because the w is work done by the surroundings (system does work to expand)
Delta U = q+w
our w value would be negative because the w is work done by the surroundings (system does work to expand)
- Thu Feb 01, 2018 3:58 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Adiabatic [ENDORSED]
- Replies: 4
- Views: 532
Re: Adiabatic [ENDORSED]
q=0 because heat is neither released nor absorbed by the system. Therefore, the only energy transfer is done by work (w).
- Sat Jan 27, 2018 12:30 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Entropy Units
- Replies: 2
- Views: 784
Re: Entropy Units
Entropy is also represented by the equation
deltaS= Qrev/T
Therefore the units of Entropy are J * K-1
deltaS= Qrev/T
Therefore the units of Entropy are J * K-1
- Sat Jan 27, 2018 12:24 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Entropy of gas vs liquid vs solid
- Replies: 7
- Views: 9158
Re: Entropy of gas vs liquid vs solid
Whenever a substance is being heated AND is not going through a phase change, the temperature of the substance (average Kinetic Energy of each particle) increases. As the total kinetic energy of the particles of the substance increases, entropy increases. Therefore, because a gas has a higher temper...
- Sat Jan 27, 2018 12:10 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Systems in practice
- Replies: 4
- Views: 534
Re: Systems in practice
One can argue that the universe is an isolated system because matter and energy is neither created nor destroyed.
- Fri Jan 19, 2018 4:52 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: atm*L to J
- Replies: 1
- Views: 912
Re: atm*L to J
101.325 is the conversion of kPa to atm (101.325 kPa = 1 atm). 1 Joule = 1 Pa * m3 Work = Pressure * Volume For irreversible expansion W=-P ex * Delta V For this system, we would use 101.325 kPa to convert our units from atm to Pa. (Then we would convert our liters to cubic meters) Ex 8.11: w= -(1.0...
- Fri Jan 19, 2018 4:44 pm
- Forum: Calculating Work of Expansion
- Topic: 8.11
- Replies: 1
- Views: 228
Re: 8.11
101.325 is the conversion of kPa to atm (101.325 kPa = 1 atm).
1 Joule = 1 Pa*m3
1.20 L = 1.2x10-3m3
Therefore, w= -(101325 Pa)(1.2x10-3m3) = -122J
1 Joule = 1 Pa*m3
1.20 L = 1.2x10-3m3
Therefore, w= -(101325 Pa)(1.2x10-3m3) = -122J
- Fri Jan 19, 2018 4:33 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Done By The System vs. Done On the System
- Replies: 3
- Views: 307
Re: Done By The System vs. Done On the System
Work = -Pressure external * Delta V When Delta V is positive, the system expands. This means that the work done on the system is negative (system is doing work on surroundings). When Delta V is negative, the system compresses. This means that the work done on the system is positive (surroundings doi...
- Fri Jan 12, 2018 9:42 am
- Forum: Phase Changes & Related Calculations
- Topic: Constant Temperature
- Replies: 4
- Views: 430
Re: Constant Temperature
Take the vaporization of water as an example. When we boil water at 100C= 373K the heat source provides energy to break the water molecules' bonds instead of heating up the temperature of the water. Thus, the temperature of the water remains the same during a phase change.
- Fri Jan 12, 2018 9:36 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.49
- Replies: 5
- Views: 414
Re: 8.49
We use 298K for the standard temperature for thermodynamics because 298K = 25C which is room temperature
- Thu Jan 11, 2018 10:28 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Ch 8 #57
- Replies: 2
- Views: 253
Re: Ch 8 #57
In 8.57 each individual enthalpy value is given in kJ/mol. Thus, I believe that the question is asking us to solve for the enthalpy for the hydrogenation of 1 mole of ethane. 8.61 gives each individual enthalpy value in kJ (possibly because it is not solving for the enthalpy of 1 mole of Bromide Gas...
- Wed Dec 06, 2017 10:14 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Drawing the Lewis structure for Coordination Compounds
- Replies: 3
- Views: 499
Drawing the Lewis structure for Coordination Compounds
If we are given a coordination compound, i.e. [HgF2(OH2))2]1+, do we use the total charge of the compound (1+) to find the formal charge of the lewis structure in addition to finding the oxidation state of the metal atom?
- Wed Dec 06, 2017 10:07 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: dipole moment chapter 4 test [ENDORSED]
- Replies: 3
- Views: 983
Re: dipole moment chapter 4 test [ENDORSED]
PO 4 3- has a resonance structure which creates a uniform negative polar charge around the Phosphate (in a tetrahedral). Because the negative polar charge and strength of each Oxygen is equal, the charges cancel each other out and there is no dipole moment. PCl 5 is the same in the sense that each C...
- Thu Nov 30, 2017 3:24 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Knowing VSEPR Shapes
- Replies: 3
- Views: 605
Re: Knowing VSEPR Shapes
Here's a table of all of the VSEPR Shapes that we need to know. I hope that this helps!
- Tue Nov 28, 2017 8:20 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Question 11.45 (c)
- Replies: 1
- Views: 255
Question 11.45 (c)
The solution manual states that the dissociation of Cl2 is more stable than F2 because F2 has a larger equilibrium constant. Why does a smaller equilibrium constant make a molecule more stable?
- Fri Nov 24, 2017 2:22 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Determining whether a complex is tetrahedral or square-planar?
- Replies: 2
- Views: 1269
Re: Determining whether a complex is tetrahedral or square-planar?
Sorry I pasted twice, and I can't delete one of the images. I hope that the visual helps!
- Fri Nov 24, 2017 2:19 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Determining whether a complex is tetrahedral or square-planar?
- Replies: 2
- Views: 1269
Re: Determining whether a complex is tetrahedral or square-planar?
A tetrahedral has 4 bonds and no lone pairs.
A square planar molecular shape has an octahedral electron structure with 4 bonds and 2 lone pairs about the central atom. (see image in link)
https://image.slidesharecdn.com/10lectu ... 1381918593
A square planar molecular shape has an octahedral electron structure with 4 bonds and 2 lone pairs about the central atom. (see image in link)
https://image.slidesharecdn.com/10lectu ... 1381918593
- Fri Nov 24, 2017 2:12 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Value of K
- Replies: 1
- Views: 234
Re: Value of K
For a small value of K (K< 1x10 -3 ) then the "equilibrium sits to the left" Because K=[products]/[reactants], a small K would have a large reactant concentration (left of the equation) and a small product concentration (right of the equation) at equilibrium. i.e. if K=[0.00110M]/[2.30M], ...
- Fri Nov 17, 2017 10:17 am
- Forum: Hybridization
- Topic: Triple bond sigma vs. pi
- Replies: 3
- Views: 389
Re: Triple bond sigma vs. pi
https://www.google.com/url?sa=i&rct=j&q=&esrc=s&source=images&cd=&cad=rja&uact=8&ved=0ahUKEwj2gO_Am8bXAhXjh1QKHZevDo0QjRwIBw&url=http%3A%2F%2Fphilschatz.com%2Fchemistry-book%2Fcontents%2Fm51058.html&psig=AOvVaw06y99Sy5o2HwIBwz1IOUVH&ust=1511028916311529 We...
- Fri Nov 17, 2017 10:13 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angle
- Replies: 7
- Views: 1015
Re: Bond Angle
Hello, unless we test it experimentally, we do not know the exact angles of bonds caused by electron repulsion. Thus, we just assume that the bond angles are slightly less than 109.5 degrees for a trigonal pyramidal molecular structures.
- Thu Nov 09, 2017 11:56 am
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: delta v
- Replies: 4
- Views: 609
Re: delta v
yes for example if our velocity is 20m/s \pm 5m/s our velocity would range from 15m/s to 25m/s. Delta v is the range of the velocity. This is found subtracting the upper and lower range of the velocity (25m/s-15m/s= 10m/s ). We can use the shortcut of multiplying the velocity found after the \pm (5m...
- Thu Nov 09, 2017 11:47 am
- Forum: Trends in The Periodic Table
- Topic: Noble Gases
- Replies: 3
- Views: 1715
Re: Noble Gases
If we were to try to add or subtract an electron from a noble gas, it would require an extensive amount of energy.
- Thu Nov 02, 2017 10:48 pm
- Forum: Bond Lengths & Energies
- Topic: Bond Length
- Replies: 5
- Views: 795
Re: Bond Length
First we must find the total number of electrons in the molecule. I usually make everything a single bond first and then adjust accordingly by adding double/triple bonds if I have too many electrons (more than the calculated total number of electrons that belongs in the molecule). Then we would calc...
- Thu Nov 02, 2017 10:38 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Uncertainty Principle [ENDORSED]
- Replies: 5
- Views: 707
Re: Uncertainty Principle [ENDORSED]
Also if you use number 1.45 as a practice problem, the solutions manual is incorrect. The actual velocity given is 5.00 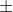 5.0m/s
5.0m/s
Thus,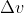 =10.0m/s NOT 5.0m/s as the solution manual incorrectly states. Therefore, your answer is
=10.0m/s NOT 5.0m/s as the solution manual incorrectly states. Therefore, your answer is 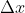 =6.7x10-37m
=6.7x10-37m
Thus,
- Thu Oct 26, 2017 4:19 pm
- Forum: Ionic & Covalent Bonds
- Topic: covalent bond
- Replies: 7
- Views: 1172
Re: covalent bond
Some examples of molecules that use covalent bonds are
Bromine (Br2)
Iodine (I2)
Nitrogen (N2)
Chlorine (Cl2)
Hydrogen (H2)
Oxygen (O2)
Fluorine (F2)
Carbon Dioxide (CO2)
Methane (CH4)
Water (H2O)
Bromine (Br2)
Iodine (I2)
Nitrogen (N2)
Chlorine (Cl2)
Hydrogen (H2)
Oxygen (O2)
Fluorine (F2)
Carbon Dioxide (CO2)
Methane (CH4)
Water (H2O)
- Thu Oct 26, 2017 4:14 pm
- Forum: Trends in The Periodic Table
- Topic: Electron Affinity
- Replies: 2
- Views: 295
Re: Electron Affinity
Electronegativity is an atom's numerical value which measures its ability to form a covalent bond. Electron affinity is the amount of energy that an atom releases when an electron is added to it. Additionally, Electron affinity is a given value for each atom, but electronegativity varies based on di...
- Thu Oct 19, 2017 4:00 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Homework Problem 1.15
- Replies: 4
- Views: 611
Re: Homework Problem 1.15
We assume that n=1 because this is a Lyman Series, and the lowest possible energy level is n=1.
For Balmer, the lowest energy level is n=2
For Paschen n=3
For Brackett n=4
For Pfund n=5
For Balmer, the lowest energy level is n=2
For Paschen n=3
For Brackett n=4
For Pfund n=5
- Thu Oct 19, 2017 3:56 pm
- Forum: Properties of Light
- Topic: the quantum world
- Replies: 7
- Views: 853
Re: the quantum world
Also, know which types of wavelengths correspond to higher or lower frequency.
i.e. x-rays have short wavelengths and therefore have a higher frequency than radio waves which have long wavelengths.
i.e. x-rays have short wavelengths and therefore have a higher frequency than radio waves which have long wavelengths.
- Thu Oct 12, 2017 5:31 pm
- Forum: Photoelectric Effect
- Topic: Mass of an electron
- Replies: 4
- Views: 578
Re: Mass of an electron
Yes, for the mass of an electron use kg
Equation: E(kinetic)=(1/2)me-(ve-)2
Units: Joules = kg (m2/s2)
Equation: E(kinetic)=(1/2)me-(ve-)2
Units: Joules = kg (m2/s2)
- Thu Oct 12, 2017 5:08 pm
- Forum: Photoelectric Effect
- Topic: Photoelectric effect example problem [ENDORSED]
- Replies: 2
- Views: 605
Re: Photoelectric effect example problem [ENDORSED]
Note the Photoelectric Effect equation: E (Photon) - Work Function = E (Kinetic) . E (Kinetic) = 1/2 (m e- v e- 2 ) The equation for E (Photon) = h v (Plank's Constant * Frequency) We know that the equation for the speed of light is: C= Wavelength * Frequency. Therefo...
- Thu Oct 05, 2017 10:44 am
- Forum: General Science Questions
- Topic: Sig Figs
- Replies: 9
- Views: 1819
Re: Sig Figs
In addition, if we have to convert our calculated number of moles into grams, do not round off the number of moles. We only round to our number of significant figures at the very end of the problem. i.e. Say we are limited to 3 sig figs, and we calculated 3.45654 moles of Oxygen gas. We would multip...
- Thu Oct 05, 2017 10:30 am
- Forum: Balancing Chemical Reactions
- Topic: Combustion Question
- Replies: 9
- Views: 1455
Re: Combustion Question
In addition, Oxygen must be present in order for a combustion reaction to occur. In most cases, Oxygen is in excess because it is found in the atmosphere. i.e. When we burn Butane in an outdoor barbecue, we have an excess Oxygen.

