Search found 36 matches
- Sat Mar 17, 2018 11:02 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Friday Section Test 2
- Replies: 2
- Views: 414
Re: Friday Section Test 2
You can also think of the reducing agent as the one that is being oxidized.
- Sat Mar 17, 2018 10:57 pm
- Forum: *Alkanes
- Topic: The symbol R
- Replies: 5
- Views: 1553
Re: The symbol R
When writing more than one R, do we have to denote them differently? For example, would we use R, R', R''...
- Sat Mar 17, 2018 10:54 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Arrhenius Graph
- Replies: 2
- Views: 1045
Re: Arrhenius Graph
Yes, these will be the axes b/c it will result in a straight line with a negative slope. The slope of the line is equal to the activation energy/R. You can think of it as being in the slope-intercept form y=mx+b
- Sat Mar 17, 2018 10:52 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Using Appendix 2A
- Replies: 2
- Views: 527
Re: Using Appendix 2A
For our test, I think we'll have to reverse the half reaction of the anode (where oxidation occurs) to get a cell potential that will result in a positive value.
- Sat Mar 17, 2018 10:49 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Standard enthalpy change
- Replies: 2
- Views: 483
Standard enthalpy change
Can someone explain why the deltaH was multiplied by 2 mols?
- Sat Mar 17, 2018 7:55 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Elementary Reaction
- Replies: 2
- Views: 453
Re: Elementary Reaction
An elementary step's rate law must agree with the observed rate law of the overall reaction. The step whose rate law agrees is going to be the slowest step.
- Sat Mar 17, 2018 7:35 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Test 2 #5a
- Replies: 1
- Views: 308
Re: Test 2 #5a
Would you mind posting the question? It'd be easier on us so that we don't have to refer to it
- Sat Mar 17, 2018 5:50 pm
- Forum: Balancing Redox Reactions
- Topic: Notation question
- Replies: 2
- Views: 515
Notation question
given the balanced equation: NaN3 --> 3N2+2Na
Can someone explain how N is oxidized? The solution explains it in the screenshot I attached, but uses a notation I'm not familiar with. Explanation?
Can someone explain how N is oxidized? The solution explains it in the screenshot I attached, but uses a notation I'm not familiar with. Explanation?
- Sat Mar 17, 2018 2:00 am
- Forum: Phase Changes & Related Calculations
- Topic: Sign for compression vs expansion
- Replies: 4
- Views: 1135
Re: Sign for compression vs expansion
Yes, that is correct. You can assume pressure is 1 atm and then plug in values for delta V to check. If you compress something, Vf-Vi will be negative, so the two negative signs will combine to make a positive value. Delta V is positive for expansion, so the value will end up being negative.
- Thu Mar 15, 2018 9:11 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Please explain k'
- Replies: 3
- Views: 486
Please explain k'
When Lavelle started lecturing on reaction mechanisms, he gave the equation A+B+C–>P He said that if the initial concentrations of both B and C were a lot greater than the initial concentration of A, then the rate=k' [A]^{n} since B and C remain essentially constant. I'm confused as to why we are us...
- Fri Feb 23, 2018 5:40 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Galvanic Cells
- Replies: 5
- Views: 678
Re: Galvanic Cells
If we calculate a standard potential and the value comes out to be negative, does that mean that it's not a galvanic cell? If not, then what type of cell is it?
- Fri Feb 23, 2018 5:38 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Standard State with Nernst Equation
- Replies: 4
- Views: 595
Re: Standard State with Nernst Equation
I believe that the simplified equation of E=Enot-RT/nF*lnQ, which is E=Enot-0.00591/n * log Q, is given to us specifically so that we don't have to plug in values for T, since it's simplified for use under STP.
- Fri Feb 23, 2018 5:27 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: signs
- Replies: 6
- Views: 1133
Re: signs
Wouldn't it depend on what your initial concentrations are? I would think that if you start with more products, they would decrease over time, and your rate for the reverse rxn would be (-) negative. Is this correct?
- Fri Feb 23, 2018 5:17 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagrams [ENDORSED]
- Replies: 7
- Views: 860
Re: Cell Diagrams [ENDORSED]
If you notice, the inert electrodes are usually denoted as solids by (s), as most metals are solid at STP, and they have conductive properties, which is why they're useful as inert electrodes. There are exceptions, though. For ex, mercury is a liquid at STP, so it'd be written as Hg(l) in the cell d...
- Fri Feb 23, 2018 5:01 pm
- Forum: General Rate Laws
- Topic: Rate Constant K [ENDORSED]
- Replies: 4
- Views: 658
Re: Rate Constant K [ENDORSED]
You say that k varies depending on activation energy and temperature, but how exactly would we go about calculating the value of k?
- Fri Feb 23, 2018 4:53 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Initial rates
- Replies: 5
- Views: 3259
Re: Initial rates
To explain why we compare initial rates, we can refer to the following equation that Lavelle used in class: NH_{4}^{+}(aq)+NO_{2}^{-}(aq)\rightarrow N_{2}(g)+H_{2}O(l)) Since N2 is a gas, it leaves the solution. As a result the reverse reaction is less likely to o...
- Tue Feb 13, 2018 5:37 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Criterion for Assessing Spontaneity of a Reaction
- Replies: 1
- Views: 205
Re: Criterion for Assessing Spontaneity of a Reaction
Sorry. The fourth letter should have been
d)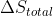
d)
- Tue Feb 13, 2018 5:22 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Criterion for Assessing Spontaneity of a Reaction
- Replies: 1
- Views: 205
Criterion for Assessing Spontaneity of a Reaction
How would you go about answering the following question? Under what conditions, if any, does the sign of each of the following quantities provide a criterion for assessing the spontaneity of a reaction? (a) \Delta G^{\circ}< 0 ; (b) \Delta H^{\circ} ; (c) \Delta S^{\circ} ; (d) \Delta S^{\circ}
- Fri Feb 02, 2018 2:41 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Bulk Matter
- Replies: 1
- Views: 295
Re: Bulk Matter
The book defines bulk matter as "matter composed of large numbers of atoms." It probably means everyday objects like a piece of wood.
- Fri Feb 02, 2018 2:37 am
- Forum: Phase Changes & Related Calculations
- Topic: Enthalpy vs. Entropy
- Replies: 4
- Views: 545
Re: Enthalpy vs. Entropy
Would you mind explaining why enthalpy can be measured as an amount altogether but entropy can only be measured by the changes in it?
- Fri Feb 02, 2018 2:35 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: entropy to surroundings
- Replies: 3
- Views: 476
Re: entropy to surroundings
Because the entropy lost by the reaction goes to the surroundings, and the surroundings are gaining the entropy, this means that the entropy gained by the surroundings is equal to that lost by the reaction. If the entropy for the reaction decreased by 1 J/K (-1 J/K), for example, then the entropy of...
- Fri Feb 02, 2018 2:25 am
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: 9.43
- Replies: 3
- Views: 423
Re: 9.43
I also don't understand this question. Are we supposed to convert the grams of water to moles and use the molar heat capacity instead? I see that it says constant pressure, and that almost lead me to believe that I needed to use 5/2R for heat capacity. However, given that it's a liquid, doesn't that...
- Fri Feb 02, 2018 1:45 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Question about equation?
- Replies: 3
- Views: 376
Re: Question about equation?
By definition, an ideal gas is a gas made of point particles (zero dimensional/no volume particles). Therefore, treating any real gas as an ideal gas is always an approximation. While the approximation is closer for monatomic gases (He, Ne, etc.), we can apply it to any real gas. The heat capacity t...
- Fri Feb 02, 2018 1:24 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: bond enthalpy [ENDORSED]
- Replies: 4
- Views: 581
Re: bond enthalpy [ENDORSED]
I'm not sure Lavelle has covered this in lecture, but I would say that polarity has nothing to do bond enthalpies.
- Fri Feb 02, 2018 1:18 am
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Extensive/Intensive Property [ENDORSED]
- Replies: 4
- Views: 605
Re: Extensive/Intensive Property [ENDORSED]
To add to the above response, you can also consider heat capacity vs. specific heat capacity. The heat capacity of a bomb calorimeter, for example, does not depend on the amount of substance because it is a defined object. It has a specified mass for which to measure it's heat capacity, and we canno...
- Tue Jan 30, 2018 10:13 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Cv vs. R
- Replies: 1
- Views: 311
Re: Cv vs. R
When you are measuring the change in heat, or \Delta H , of an ideal gas at constant volume, the specific heat will be equal to 3/2 times the ideal gas constant. Here is the equation: C_{V}=3/2*R Therefore, when measuring \Delta H , you replace C_{V} with 3/2*R in your \Delta H=mC_{V}\Delta T equati...
- Sat Jan 20, 2018 2:25 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: J vs. kJ [ENDORSED]
- Replies: 4
- Views: 437
Re: J vs. kJ [ENDORSED]
Typically, you will determine if you need to use J or kJ based on the question. The question may give you a value in kJ, but it can still ask for your answer to use J instead, and vice versa, so all you'd need to do is make the conversion.
- Sat Jan 20, 2018 2:22 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: standard heat of combustion vs. standard heat of formation
- Replies: 2
- Views: 887
Re: standard heat of combustion vs. standard heat of formation
The standard heat of combustion will always be negative since the reaction will be giving off heat as it breaks the chemical bonds. The heat of formation should always be positive since it will be taking in heat in order to produce the chemical bonds. They can be related to one another by using the ...
- Sat Jan 20, 2018 2:00 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: ∆H equation
- Replies: 3
- Views: 551
Re: ∆H equation
Isn't the equation for finding change in heat as follows: 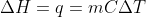
I know that the m accounts for mass instead of moles(which is usually represented as n), so would we simply switch out the m for n if we are presented with molar heat capacity?
I know that the m accounts for mass instead of moles(which is usually represented as n), so would we simply switch out the m for n if we are presented with molar heat capacity?
- Sat Dec 09, 2017 8:43 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: X in ICE table
- Replies: 3
- Views: 3618
Re: X in ICE table
If you were given the concentrations to both products and reactants, you would first need to find Q, which is given by [P]/[R]. Given a value for Kc, you would need to compare Q to Kc and see if it is greater than or less than. If Q is greater, then that means that the reaction would shift toward th...
- Thu Dec 07, 2017 12:53 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Oxidation Numbers
- Replies: 2
- Views: 395
Re: Oxidation Numbers
Oxidation numbers are the total sum of ionic charges for a compound. For example, if we were looking at water, H20, we can see that it has two elements that compose it: 1) hydrogen, which is usually found as H+, so it has a positive charge of one and 2)oxygen, which is usually found as O 2- . If you...
- Thu Dec 07, 2017 10:21 am
- Forum: Amphoteric Compounds
- Topic: Amphoteric vs amphiprotic
- Replies: 2
- Views: 1708
Re: Amphoteric vs amphiprotic
Amphoteric refers to the ability of X, a compound, to act as both a base and an acid. For example, in the chemical equation H_{2}O(l)+H_{2}O(l)\rightarrow H_{3}O^{+}(aq)+OH^{-}(aq) , water acts as both an acid (whose conjugate base is hydroxide, OH-) and as a base (wh...
- Thu Oct 26, 2017 11:16 pm
- Forum: Lewis Structures
- Topic: S and P blocks and Lewis structures [ENDORSED]
- Replies: 4
- Views: 1524
Re: S and P blocks and Lewis structures [ENDORSED]
Lewis structures are used to model the chemical bonds between two or more elements regardless of what block they are in. Lewis models use the valance electrons(v.e-) of elements to visualize the bonds because the v.e- are in the outermost shell, and it wouldn't make sense for elements to bond with e...
- Thu Oct 26, 2017 5:09 pm
- Forum: Trends in The Periodic Table
- Topic: Transition Metals
- Replies: 3
- Views: 496
Re: Transition Metals
Transition metals are those elements in the d-Block that consists entirely of metals. Those metals always form cations (ions with a positive charge) and the charges in the metals will vary. The reason they all form cations is because transition metals have relatively low ionization energies, so an e...
- Fri Oct 13, 2017 4:44 pm
- Forum: Photoelectric Effect
- Topic: Lyman Series v Balmer series
- Replies: 2
- Views: 395
Lyman Series v Balmer series
Can someone explain the difference between the Lyman and Blamer series to me? I couldn't really determine the difference when looking at the graphs Lavelle showed us during lecture. Fundamental difference would be great, and further detail would be much appreciated!
- Fri Oct 13, 2017 3:53 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: why is Bohr Frequency equation negative
- Replies: 4
- Views: 1861
Re: why is Bohr Frequency equation negative
Different elements have different characteristics, so the energy at the ground state of an electron (e-) for Hydrogen (H) is going to be different than the energy for the ground state of an e- of Helium (He), for example. The energy for a hydrogen e- at the ground state is 2.178 x 10^-20 Joules (J),...

