Search found 80 matches
- Sat Mar 17, 2018 8:53 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 14.15a
- Replies: 2
- Views: 355
Re: 14.15a
I believe it is because the Ag(s) is acting as an electrode. Although they are both solids, it would be difficult to tell which is the electrode if you just put a comma as they are both solids, so they put a line to differentiate between the electrode and the "solution". How do we know th...
- Sat Mar 17, 2018 6:46 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 14.15a
- Replies: 2
- Views: 355
14.15a
According to my understanding of Galvanic cell models, if two elements/molecules are in the same phase, they are put together between | | and separated by commas. However, the answer to this problem is Ag(s) | AgBr(s) | Br - (aq) || Ag + (aq) | Ag(s) Why isn't it Ag(s) , AgBr(s) | Br - (aq) || Ag + ...
- Sat Mar 17, 2018 5:32 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 14.13 d
- Replies: 2
- Views: 473
Re: 14.13 d
How are we supposed to know that the oxidation reaction is Au3+(aq) + 3e- --> Au(s) if, in the reaction given Au and Au3+ are on the same side (products)?
- Sat Mar 17, 2018 5:21 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagram, similar phase ordering
- Replies: 1
- Views: 319
Cell Diagram, similar phase ordering
When writing a cell diagram, if two elements/molecules are in the same container (anode or cathode) and are in the same phase, does it matter which is written first? For example, in this reaction Ce 4+ (aq) + I - (aq) --> I 2 (s) + Ce 3+ (aq) Does it matter if I write this Pt(s) | I 2 (s) | I - (aq)...
- Sat Mar 17, 2018 5:18 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 14.13 B
- Replies: 3
- Views: 453
Re: 14.13 B
Do you think on a test, they would expect us to already know that I2 is a halogen and can't conduct electricity, and thus we need Pt(s), or do you think they'd explicitly mention that on a test?
- Sat Mar 17, 2018 4:06 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Creating Cell Diagrams
- Replies: 1
- Views: 318
Creating Cell Diagrams
I'm still having some trouble figuring out how to create a cell diagram. For example, in Test 2 #6, we were given a question about it. The question said: The following redox couple forms a galvanic cell which generates a current under standard conditions. O 3 /O 2 , OH - and O 3 , H + /O 2 (a) Ident...
- Sat Mar 17, 2018 3:56 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Relationship between Cell Component and Cell Potential
- Replies: 1
- Views: 356
Relationship between Cell Component and Cell Potential
If given two reactions, is there a way to determine which reaction will go on the anode side vs. the cathode side by comparing their \Delta E ? And the \Delta E values I'm referring to would be determined using a chart like Appendix 2B. In other words, does a stronger (more positive) cell potential ...
- Sat Mar 17, 2018 2:34 pm
- Forum: Balancing Redox Reactions
- Topic: Homework Problem 14.1
- Replies: 2
- Views: 513
Homework Problem 14.1
The equation that we're supposed to balance is H + (aq) + Cr 2 O 7 2- (aq) + C 2 H 5 OH(aq) --> Cr 3+ (aq) + C 2 H 4 O(aq) + H 2 O(l) According to the solution manual, the balanced oxidation half-reaction should be C 2 H 5 OH(aq) --> C 2 H 4 O(aq) + 2e - + 2H + (aq) I'm having difficulty figuring ou...
- Sat Mar 17, 2018 2:56 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Total entropy change determining spontaneity
- Replies: 3
- Views: 641
Total entropy change determining spontaneity
I was working on homework problem 9.73, and it asks how \Delta S TOT could help assess spontaneity, and according to the solutions manual, the total change in entropy has to be positive. Can someone explain why, both mathematically and logically, a positive change in entropy signifies spontaneity? T...
- Sat Mar 17, 2018 1:25 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Entropy of Surroundings vs. System
- Replies: 2
- Views: 566
Entropy of Surroundings vs. System
When can you tell if the entropy of the surroundings/system is supposed to be positive/negative? Like in problem 9.45, it asks to find \Delta S of the systems and surroundings for the following situations: (a) vaporization of 1.00 mol CH 4 (l) at its normal BP (b) melting of 1.00 mol C 2 H 5 OH(s) a...
- Fri Mar 16, 2018 11:51 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Change in Entropy Equations
- Replies: 2
- Views: 512
Re: Change in Entropy Equations
Then how come, for example, in question 9.15, which asks to calculate the entropy change for (a) the freezing of 1.00 mol H2O(l) at 0.00 C and the vaporization of 50.0g of ethanol at 531.5K, the solutions manual uses the equation 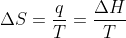 ? Why isn't it negative?
? Why isn't it negative?
- Fri Mar 16, 2018 8:25 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: 9.1
- Replies: 5
- Views: 951
Re: 9.1
Hi! Because it asks for the rate your body generates heat "in your surroundings" the formula has a -q. This because the system is releasing heat into the surroundings, or heat is flowing out of the system (with the system being the body). That makes sense, but then why is the final answer...
- Fri Mar 16, 2018 8:14 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Change in Entropy Equations
- Replies: 2
- Views: 512
Change in Entropy Equations
I'm having difficulty differentiating between when to use the equation 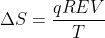 and
and 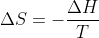 .
.
If someone could please explain, that would be appreciated.
Thanks!
If someone could please explain, that would be appreciated.
Thanks!
- Fri Mar 16, 2018 8:06 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: 9.1
- Replies: 5
- Views: 951
9.1
I guess this just proves how much I've forgotten about old material, but I'm having trouble on 9.1 The question asks the find at what rate does your body heat generate entropy in your surroundings, taken to be at 20. C? The body generates heat at a rate of about 100. W (J/s) I can't seem to figure o...
- Fri Mar 16, 2018 6:51 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.99
- Replies: 1
- Views: 346
8.99
The question asks to find the final temperature of a solution when a piece of zinc of mass 8.5g is dropped into an apparatus containing 800.0mL of 0.500M HCl(aq). You're supposed to assume that the density and molar heat capacity of the HCl solution are the same as those of water. When looking at th...
- Thu Mar 15, 2018 9:23 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Class Study Google Doc
- Replies: 5
- Views: 990
Class Study Google Doc
Hey everyone, I made a Google Doc Study Sheet that everyone can access via their UCLA emails (@g.ucla.edu). It has all the main topics listed by Dr. Lavelle in his chapter outlines and a section for other info for each chapter. I'll be adding to it as I go through studying (which is why it's empty r...
- Wed Mar 07, 2018 9:42 pm
- Forum: General Rate Laws
- Topic: 15.23 b
- Replies: 3
- Views: 482
Re: 15.23 b
I used the equation 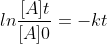 and got the same answer as the solutions manual. I think the two equations are equivalent because of the negative sign I used in my answer, when transferred to the other side, would flip the two concentrations.
and got the same answer as the solutions manual. I think the two equations are equivalent because of the negative sign I used in my answer, when transferred to the other side, would flip the two concentrations.
- Wed Mar 07, 2018 9:40 pm
- Forum: General Rate Laws
- Topic: Integrated Rate Laws
- Replies: 6
- Views: 825
Re: Integrated Rate Laws
The derived rate laws are not on the constants sheet, and as Lavelle emphasizes, he never asks specifically the steps for derivation, but I assume it would still be helpful to understand the derivations and at least memorize the derived formulas and how they are used.
- Wed Mar 07, 2018 5:41 pm
- Forum: General Rate Laws
- Topic: Reaction rates
- Replies: 3
- Views: 499
Re: Reaction rates
The unique average rate can be applied to any of the molecules in the reaction by multiplying it by the coefficient in front of the molecule, if that helps!
- Wed Mar 07, 2018 5:32 pm
- Forum: General Rate Laws
- Topic: Test 3 material
- Replies: 6
- Views: 768
Re: Test 3 material
Adrian Lim 1G wrote:Does anyone know which homework problem 15.6 would go up to?
I believe it is up to question 39
- Mon Mar 05, 2018 9:27 pm
- Forum: General Rate Laws
- Topic: 15.39b
- Replies: 3
- Views: 555
15.39b
The question asks to find the amount of time it would take in the reaction of A --> 2B + C, when [A] 0 = 0.15M, for the concentration of B to increase to 0.19M, given that k = 0.0035L/mol*min in the rate law for the loss of A. I was wondering why the solutions manual solves this equation as a second...
- Mon Mar 05, 2018 9:04 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: derivations
- Replies: 2
- Views: 401
Re: derivations
As always, I don't believe we'll need to know the specific steps of the derivations, but it is always helpful to understand how to get from one equation to another. However, the derived equations (I believe, please correct me if I'm wrong) are not included in the constants sheet.
- Sun Mar 04, 2018 9:36 pm
- Forum: General Rate Laws
- Topic: Test #3
- Replies: 1
- Views: 285
Test #3
I was wondering which textbook questions would correspond to the test this upcoming week so I know which ones to focus on and practice. Thanks!
- Thu Feb 22, 2018 10:43 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Homework 14.25
- Replies: 4
- Views: 606
Homework 14.25
The question asks to put the following metals in order of increasing strength as reducing agents for species in aqueous solutions. Cu, Zn, Cr, Fe According to the solutions manual, Zn is weaker than Cr. When looking at appendix 2B, the E o of Cr 3+ + 3e - —> Cr is -0.74 V. However the E o of Cr 2- +...
- Thu Feb 22, 2018 9:26 pm
- Forum: Balancing Redox Reactions
- Topic: 14.17
- Replies: 3
- Views: 1110
Re: 14.17
What happens in this reaction to the potassium and the chloride though? And are we supposed to assume that permanganate will always dissociate into Mn2+ and Fe2+ into Fe3+?
- Thu Feb 22, 2018 9:01 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 14.13c
- Replies: 4
- Views: 468
14.13c
The question asks to write cell diagram for this equation Cl 2 (g) + H 2 (g) —> HCl(aq) According to the solutions manual, it looks like Pt(s)|H 2 (g)|H + (aq)||Cl - (aq)|Cl 2 (g)|Pt(s) I’m confused as to why, on the cathode side, Cl - comes before Cl 2 if, according to the reaction, Cl 2 is the rea...
- Thu Feb 22, 2018 8:05 pm
- Forum: Balancing Redox Reactions
- Topic: Reaction E [ENDORSED]
- Replies: 5
- Views: 716
Reaction E [ENDORSED]
How come when figuring out the reaction E, we don’t manipulate the potentials? For example, for homework problem 14.11, the question involves the half reactions oh Ni(s) + 2e - —> Ni with E = -0.23V and Ag + + e - —> Ag with E = +0.80V. In order to make the reaction, we must reverse the first reacti...
- Tue Feb 20, 2018 2:21 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation state
- Replies: 7
- Views: 1000
Re: Oxidation state
There are a couple standard rules that will help determine the oxidation states of certain atoms in molecules. Hydrogen always has a +1 oxidation state. Oxygen almost always has a -2 oxidation state (unless its with H 2 O 2 ). Let's find out the oxidation state of oxygen in H 2 O 2 as an example. We...
- Mon Feb 19, 2018 10:47 pm
- Forum: Balancing Redox Reactions
- Topic: 14.5 d steps
- Replies: 2
- Views: 528
Re: 14.5 d steps
This is what I did for the entire question: P 4 (s) --> H 2 PO 2 - (aq) + PH 3 (g) 0 +1 +1 -2 -3 +1 (respective oxidation states of the atoms as they appear in the equation For the oxidation half reaction, we have P 4 (s) --> 4H 2 PO 2 - (aq) I added the coefficient 4 in front of H 2 PO 2 - because ...
- Mon Feb 19, 2018 9:55 pm
- Forum: Balancing Redox Reactions
- Topic: 14.5a
- Replies: 4
- Views: 546
Re: 14.5a
The atoms being oxidized and reduced actually aren't both present in the same molecule in the products. The oxidation half reaction is O3 --> O2 and the reduction half reaction is Br- --> BrO3- I don't understand how O 3 is being reduced to O 2 if the oxidation state of both oxygen atoms are 0.
- Mon Feb 19, 2018 9:35 pm
- Forum: Balancing Redox Reactions
- Topic: H+ or H3O+
- Replies: 2
- Views: 366
Re: H+ or H3O+
To expand,
It doesn't matter, but if you add H3O+ we have to continue to balance the reaction with more H2O molecules because of the oxygen atoms present.
Thus, in order to save time, it's easier to just add H+ ions to balance out the presence of H atoms.
It doesn't matter, but if you add H3O+ we have to continue to balance the reaction with more H2O molecules because of the oxygen atoms present.
Thus, in order to save time, it's easier to just add H+ ions to balance out the presence of H atoms.
- Mon Feb 19, 2018 9:34 pm
- Forum: Balancing Redox Reactions
- Topic: Balanced Redox Reactions
- Replies: 2
- Views: 416
Re: Balanced Redox Reactions
To expand on that answer: You only add H 2 O when you need to balance out the oxygens in an equation. In the end (your answer), it doesn't matter which side the water molecules are on. We can add these water molecules because we assume that all these reactions occur in aqueous solutions, and so wate...
- Mon Feb 19, 2018 9:30 pm
- Forum: Balancing Redox Reactions
- Topic: 14.5a
- Replies: 4
- Views: 546
14.5a
Can someone please walk me through how to do this problem?
It's asking to balance the equation O3(aq) + Br-(aq) --> O2(g) + BrO3-.
I'm just confused on how to balance this when the atoms being oxidized and reduced are both present in the same molecule in the products.
It's asking to balance the equation O3(aq) + Br-(aq) --> O2(g) + BrO3-.
I'm just confused on how to balance this when the atoms being oxidized and reduced are both present in the same molecule in the products.
- Mon Feb 19, 2018 9:27 pm
- Forum: Balancing Redox Reactions
- Topic: example 14.1 in the book
- Replies: 1
- Views: 336
Re: example 14.1 in the book
In C 2 H 2 O 4 , we know that the H has an oxidation state of +1 (hydrogen always has an oxidation state of +1, unless I'm mistaken) and the O has an oxidation state of -2 (oxygen almost always has an oxidation state of -2, unless in situations as H 2 O 2 , where it has an oxidation state of -1, but...
- Wed Feb 14, 2018 5:07 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Enthalpy of fusion [ENDORSED]
- Replies: 4
- Views: 1184
Re: Enthalpy of fusion [ENDORSED]
We use the equation q=mC\Delta T+m\Delta H to find the q ice cream because we have to account for the fact that it changes from solid (frozen) to liquid (melted). The mC\Delta T accounts for the ice cream changing and then m\Delta H accounts for the phase change, where \Delta H=\Delta Hfusion .
- Wed Feb 14, 2018 5:03 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Practice Midterm #7
- Replies: 2
- Views: 319
Re: Practice Midterm #7
Make sure you account for the phase change of the ice to liquid in your qice
- Wed Feb 14, 2018 1:07 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Difference between delta S and delta S naught
- Replies: 2
- Views: 8272
Re: Difference between delta S and delta S naught
The third law of thermodynamics says that the total entropy is always increasing, so delta S total must be positive. The standard enthalpy for a reaction can be positive or negative depending on the reaction. For example, building polymers from monomers has a negative delta S (the complexity is inc...
- Wed Feb 14, 2018 12:58 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Isothermal/Isobaric/Irreversible/Reversible/Other Topics Covered
- Replies: 3
- Views: 525
Re: Isothermal/Isobaric/Irreversible/Reversible/Other Topics Covered
Can anyone else list any more?
- Wed Feb 14, 2018 10:49 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Midterm Review #4 Entropy and Work Calculations [ENDORSED]
- Replies: 1
- Views: 338
Midterm Review #4 Entropy and Work Calculations [ENDORSED]
Can someone please walk me through how to find \Delta S for the reaction? The question goes: You have a system consisting of 0.60 moles of an ideal gas contained in a 50.0L container at 1.0 atm. You just love chemistry to a fault, so you perform a series of steps to the system. First, you perform an...
- Wed Feb 14, 2018 10:00 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Isothermal/Isobaric/Irreversible/Reversible/Other Topics Covered
- Replies: 3
- Views: 525
Isothermal/Isobaric/Irreversible/Reversible/Other Topics Covered
I'm trying to come up with a composite list of all the special situations that can occur under certain conditions. For example, I believe that under isothermal conditions, \Delta U = 0 . If anyone could add to this list, that would be appreciated! And also, perhaps adding an explanation as to why ea...
- Tue Feb 13, 2018 10:15 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: (DeltaU)=q+w Isothermal
- Replies: 2
- Views: 377
Re: (DeltaU)=q+w Isothermal
For isothermal reactions, 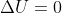 and so
and so 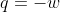
- Tue Feb 13, 2018 10:06 pm
- Forum: Calculating Work of Expansion
- Topic: Reversible reaction and Detla U
- Replies: 3
- Views: 516
Re: Reversible reaction and Detla U
Are you referring to delta u in an isothermal reaction? This is because for an ideal gas U = 3/2 nRT. Therefore, if the moles of gas stay the same, n is constant, R is the gas constant, and if T is constant (which is what isothermal means) then U is constant which means delta U = 0. Sorry, do you m...
- Tue Feb 13, 2018 9:46 pm
- Forum: Calculating Work of Expansion
- Topic: Reversible vs irreversible
- Replies: 4
- Views: 459
Re: Reversible vs irreversible
I believe Kelly is talking about the direction of the arrows. If it is a double-sided arrow (like in an equilibrium reaction), it is reversible. If it is one-sided, it is irreversible.
- Tue Feb 13, 2018 6:09 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Degeneracy (W) [ENDORSED]
- Replies: 8
- Views: 1281
Re: Degeneracy (W) [ENDORSED]
Yes! As seen through stoichiometry.
- Tue Feb 13, 2018 6:02 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Useful Summary of Thermodynamic Definitions
- Replies: 55
- Views: 18723
Re: Useful Summary of Thermodynamic Definitions
Would someone be able to briefly explain the difference between isobaric, isochoric, and isothermal? I have these definitions written down and I've read through the textbook, but I feel as though I've memorized the differences and don't fully grasp the concept. Thanks! As Dr. Lavelle explained in h...
- Tue Feb 13, 2018 5:36 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: finding heat capacity using q=cΔT
- Replies: 3
- Views: 1053
Re: finding heat capacity using q=cΔT
It is negative because the q of the calorimeter is going to be the negative of the q of the reaction, which is what we use in the equation.
Hope that makes sense.
Hope that makes sense.
- Tue Feb 13, 2018 5:14 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: calorimeter problems
- Replies: 2
- Views: 403
Re: calorimeter problems
You use this equation in problems regarding calibrating a calorimeter because there's no "mass" to take into account. Rather, it would be the specific heat of a single calorimeter.
- Tue Feb 13, 2018 4:18 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.117
- Replies: 2
- Views: 377
Re: 8.117
Yes, that's correct. Because the equation is asking for the internal energy for the production of 1.00 mol H 2 , you should divide the entire equation by 3 in order to get 1 as a coefficient of H 2 on the right-hand side. Thus, your equation becomes 1/3 CH 4 (g) + 1/3 H 2 O(g) --> 1/3 CO 2 (g) + H 2...
- Tue Feb 13, 2018 3:59 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Practice Problem
- Replies: 1
- Views: 318
Practice Problem
Can someone please walk me through the steps of this problem? You have a system consisting of 0.60 moles of an ideal gas contained in a 50.0L container at 1.0 atm. You just love chemistry to a fault, so you perform a series of steps to the system. First, you perform an isobaric compression of the co...
- Tue Feb 13, 2018 2:44 pm
- Forum: Phase Changes & Related Calculations
- Topic: Origins of Internal Energy
- Replies: 2
- Views: 330
Re: Origins of Internal Energy
I think it'll definitely to helpful to understand which types of internal energy contribute most to entropy.
- Tue Feb 13, 2018 2:43 pm
- Forum: Phase Changes & Related Calculations
- Topic: Reversible
- Replies: 5
- Views: 1138
Re: Reversible
It is also helpful to remember the reversible systems do not occur in real life. Rather, this equation is used to find the maximum work available for a system.
- Tue Feb 13, 2018 2:41 pm
- Forum: Phase Changes & Related Calculations
- Topic: HW 9.37 calculating Hvap
- Replies: 1
- Views: 307
Re: HW 9.37 calculating Hvap
I know that in some previous question, the  vap has been given if you're asked to solve for something like
vap has been given if you're asked to solve for something like 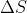 vap of a substance and also given the boiling point.
vap of a substance and also given the boiling point.
- Tue Feb 13, 2018 2:39 pm
- Forum: Phase Changes & Related Calculations
- Topic: Practice Problem
- Replies: 3
- Views: 481
Re: Practice Problem
I'm not sure where you got this problem from, but I just did it through and wanted to check my answers to see if it was correct. I got 75.84 g H 2 O. My work is as follows: 49.7g PbO \cdot \frac{1 mol PbO}{223.2 g PbO}\cdot \frac{106.9 kJ}{1 mol PbO}=23.8 kJ q=mc\Delta T -23,800J=m(4.184 J/gC...
- Tue Feb 13, 2018 2:29 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Isothermal Systems and deltaU
- Replies: 2
- Views: 314
Isothermal Systems and deltaU
I just want to clarify, that in an isothermal system/reaction, 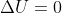 , correct?
, correct?
Thanks.
Thanks.
- Sun Feb 11, 2018 11:05 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Stability and deltaG
- Replies: 2
- Views: 498
Stability and deltaG
Just to clarify, if  is negative, the compound is stable/thermodynamically favored right? And the more negative
is negative, the compound is stable/thermodynamically favored right? And the more negative  is, the more stable the compound is.
is, the more stable the compound is.
- Fri Feb 09, 2018 9:57 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Entropy of a Irreversible Process
- Replies: 2
- Views: 440
Re: Entropy of a Irreversible Process
I agree with Justin!
The total entropy is equal to the sum of the entropy of the system and the entropy of the surroundings. Thus, when one process has a higher entropy of the surroundings, it'll have a higher total entropy than the other.
The total entropy is equal to the sum of the entropy of the system and the entropy of the surroundings. Thus, when one process has a higher entropy of the surroundings, it'll have a higher total entropy than the other.
- Fri Feb 09, 2018 9:53 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: 9.17
- Replies: 1
- Views: 323
Re: 9.17
According to Trouton's rule, the entropy of vaporization at the boiling point is approximately 85 J/kmol. This is because approximately the same increase in positional disorder occurs when any liquid is converted into vapor, so we can expect the change in entropy to be much the same in each case. Su...
- Mon Feb 05, 2018 3:37 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Reversible Reaction Total Entropy
- Replies: 1
- Views: 256
Reversible Reaction Total Entropy
Why is the total entropy of a reversible reaction = 0?
- Wed Jan 31, 2018 6:16 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: 9.43
- Replies: 5
- Views: 471
9.43
I was wondering where the solutions manual got 75.3 J/Kmol as the Cp,m of water from.
Thanks.
Thanks.
- Wed Jan 31, 2018 4:42 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Calculator Overflow? [ENDORSED]
- Replies: 1
- Views: 220
Calculator Overflow? [ENDORSED]
When trying to do 9.25 in the homework, I was trying to input (1.381\cdot 10^{-23})ln(6^{6.02\cdot 10^{23}}) in my calculator but it showed an overflow error. I'm assuming that the number was too big for my scientific calculator to handle. I'm wondering what we would do during a test...
- Wed Jan 31, 2018 4:32 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: 9.23 (molar enthalpy between molecules) [ENDORSED]
- Replies: 2
- Views: 347
9.23 (molar enthalpy between molecules) [ENDORSED]
The question asks: Which would you expect to have a higher molar entropy at T = 0, single crystals of BF 3 or of COF 2 ? Why? The answer key says COF 2 , but I don't really understand its explanation for why. Could anyone rephrase the explanation or perhaps explain it in a different way in hopes tha...
- Wed Jan 31, 2018 3:52 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: 9.7 vs 9.13
- Replies: 4
- Views: 405
9.7 vs 9.13
I'm confused about the equations that we use in 9.7 vs the equation used in 9.13. 9.7 asks to calculate the entropy change associated with raising the temperature of 1.00 mol of ideal gas atoms reversibly from 37.6 C to 157.9 C at (a) constant pressure and (b) constant volume and to assume that the ...
- Wed Jan 31, 2018 3:06 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Entropy and Temperature
- Replies: 3
- Views: 429
Entropy and Temperature
I understand that, according to \Delta S=\frac{-\Delta H}{T} , temperature and entropy are inversely related. So, an increase in temperature would result in a decrease in entropy. But I don't understand that because, how I see it, and increase in temperature results in an increase in the speed of th...
- Sun Jan 28, 2018 7:22 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Examlpe 9.6
- Replies: 2
- Views: 303
Examlpe 9.6
The question asks: Calculate the entropy of vaporization of acetone at 296 K with an external pressure of 1 bar. The molar heat capacity of liquid acetone is 127 J/Kmol, its boiling point is 329.4 K, and its enthalpy of vaporization is 29.1 kJ/mol. I understand the first two steps of finding entropy...
- Sun Jan 28, 2018 6:56 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Example 9.2
- Replies: 2
- Views: 369
Example 9.2
The question asks: A sample of nitrogen gas of volume 20.0 L at 5.00 kPa is heated from 20. C to 400. C at constant volume. What is the change in the entropy of the nitrogen? The molar heat capacity of nitrogen at constant volume, C V, m is 20.91 J/Kmol. The textbook solves this question by using \D...
- Thu Jan 25, 2018 9:12 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Examples of work being done
- Replies: 7
- Views: 1014
Examples of work being done
What are some examples in which work is being done on a system?
In an example where volume and pressure both change, is work still being done on the system?
In a system that expands against a vacuum, is work being done?
In an example where volume and pressure both change, is work still being done on the system?
In a system that expands against a vacuum, is work being done?
- Thu Jan 25, 2018 9:10 pm
- Forum: Phase Changes & Related Calculations
- Topic: enthaply
- Replies: 3
- Views: 480
Re: enthaply
You can't necessarily measure just the enthalpy (H) of a system. Rather, when we calculate the heat that is transferred during an equation, we want to compare the heat before and after the reaction has occurred, thus we would always use  in terms of measuring the enthalpy change.
in terms of measuring the enthalpy change.
- Thu Jan 25, 2018 9:03 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: DeltaU and DeltaH
- Replies: 4
- Views: 924
Re: DeltaU and DeltaH
- Sat Jan 20, 2018 4:02 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Problem 8.99
- Replies: 6
- Views: 654
Problem 8.99
The question reads: Hydrochloric acid oxidizes zinc metal in a reaction that produces hydrogen gas and chloride ions. A piece of zinc metal of mass 8.5 g is dropped into an apparatus containing 800.0 mL of 0.500 M HCl(aq). If the initial temperature of the hydrochloric acid solutions is 25C, what is...
- Sat Jan 20, 2018 2:52 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Kekule structures, Resonance, and stability
- Replies: 1
- Views: 93
Kekule structures, Resonance, and stability
Does a lowering in molar energy of a molecule mean it's more stable?
- Sat Jan 20, 2018 1:58 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Homework Problem 8.67
- Replies: 4
- Views: 225
Re: Homework Problem 8.67
So after drawing benzene without resonance, what's the difference between CRC bonds and C-C bonds?
- Sat Jan 20, 2018 1:44 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Homework Problem 8.67
- Replies: 4
- Views: 225
Re: Homework Problem 8.67
For part (a), do we just assume that anytime we use bond enthalpies, we have to subtract the enthalpy of vaporization?
And can you please show me how the molecular structure of benzene without resonance would look?
And can you please show me how the molecular structure of benzene without resonance would look?
- Sat Jan 20, 2018 1:23 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Homework Problem 8.67
- Replies: 4
- Views: 225
Homework Problem 8.67
The question asks to estimate the enthalpy of formation of compounds in their liquid states (a) H 2 O (b) CH 3 OH (c) C 6 H 6 (without resonance) (d) C 6 H 6 (with resonance) Regarding (a), I understand how to use the bond energies of H 2 and O 2 to find the formation enthalpy of H 2 O, but I don't ...
- Sat Jan 20, 2018 12:02 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Problem 8.65
- Replies: 3
- Views: 414
Problem 8.65
I was wondering why the reaction we are looking for is N 2 (g) + 5/2O 2 (g) --> N 2 O 5 (g) and why we can't solve the problem like this: 2(2NO(g) + O 2 (g) --> 2NO 2 (g)) \Delta H=2(-114.1 kJ) 4NO 2 + O 2 (g) --> 2N 2 O 5 (g) \Delta H=-110.2 kJ 4NO(g) + 2O 2 --> 4NO 2 (g) \Delta H=-228.2 kJ...
- Thu Jan 18, 2018 9:15 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Homework Question 8.47
- Replies: 4
- Views: 370
Re: Homework Question 8.47
But that ultimately give you a negative work, whereas work done ON a system should be positive???
- Thu Jan 18, 2018 8:18 pm
- Forum: Phase Changes & Related Calculations
- Topic: 8.37
- Replies: 3
- Views: 477
8.37
The question asks: (a) At its boiling point, the vaporization of 0.579 mol CH 4 (l) requires 4.76 kJ of heat. What is the enthalpy of vaporization of methane? (b) An electric heater was immersed in a flask of boiling ethanol, C 2 H 5 OH, and 22.45 g of ethanol was vaporized when 21.2 kJ of energy wa...
- Thu Jan 18, 2018 8:00 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 8.31
- Replies: 5
- Views: 657
8.31
This question explicitly asks to calculate the heat released. I was wondering how you know to find molar heat capacity (and thus you have to change the mass into number of moles, as shown in the solutions manual) rather than keeping the Kr in terms of grams.
Thanks.
Thanks.
- Fri Jan 12, 2018 11:10 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Example 8.13
- Replies: 2
- Views: 367
Example 8.13
The questions asks the estimate the enthalpy of the reaction between bromine and propene to form 1,2-dibromopropane. The enthalpy of vaporization of Br 2 is 29.9 kJ/mol, and that of CH 3 CHBrCH 2 Br is 35.61 kJ/mol. The reaction is Br 2 (l) + CH 3 CH=CH 2 --> CH 3 CHBrCH 2 Br(l) In the first step on...
- Thu Jan 11, 2018 9:42 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Constant-Pressure Calorimeter vs. Constant-Volume Bomb Calorimeter [ENDORSED]
- Replies: 1
- Views: 2333
Constant-Pressure Calorimeter vs. Constant-Volume Bomb Calorimeter [ENDORSED]
What is the difference between these two types of calorimeters and what types of calculations would each infer (i.e. if a question said that it used one or the other, what types of things should we keep in mind during our calculations)?
- Thu Jan 11, 2018 9:00 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Example 8.6
- Replies: 1
- Views: 183
Example 8.6
The question asks: Calculate the final temperature and the change in internal energy wen 500. J of energy is transferred as heat to 0.900 mol O 2 (g) at 298 K and 1.00 atm at (a) constant volume; (b) constant pressure. Treat the gas as ideal. I understand how the textbook determine C V,m and C P,m ,...

