Search found 37 matches
- Sat Mar 17, 2018 10:57 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: total entropy of the system
- Replies: 3
- Views: 589
Re: total entropy of the system
The second law does not mean that entropy always increases, but that entropy can never decrease. If total entropy is negative, the reaction is not spontaneous as you said. This means the reverse reaction is spontaneous so the total entropy increases. When the reaction is at equilibrium, the entropy ...
- Tue Mar 13, 2018 6:06 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Lecture Friday
- Replies: 4
- Views: 711
Lecture Friday
Is there a lecture on Friday?
- Mon Mar 12, 2018 9:56 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Distinguishing a Catalyst
- Replies: 4
- Views: 677
Re: Distinguishing a Catalyst
I believe the catalyst also does not appear in the overall reaction (though it may appear in the rate law).
- Mon Mar 12, 2018 9:55 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Given information
- Replies: 5
- Views: 595
Re: Given information
I believe he said in class that we would always be given the slow step and the course reader practice finals specify which step is slow as well. Another type of problem is identifying which step would be the slow step based on the rate law, in which case you naturally would not be given this informa...
- Mon Mar 12, 2018 9:53 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagram
- Replies: 3
- Views: 452
Re: Cell Diagram
Eli is right, I would disregard the last reply as I believe the species will always be solid or aqueous.
- Wed Mar 07, 2018 9:11 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: 15.27
- Replies: 2
- Views: 610
Re: 15.27
Personally (I don't have the solutions manual) I knew that to find t_{1/2} you divide ln 2 by k, so generally you divide ln(reciprocal of the fraction) by k. 15% is equivalent to the fraction 15/100 which equals 3/20, so the reciprocal is 20/3. I found the solution by taking ln(20/3) and dividing by...
- Wed Mar 07, 2018 9:09 am
- Forum: General Rate Laws
- Topic: Homework Problem 15.19C
- Replies: 5
- Views: 648
Re: Homework Problem 15.19C
Yup, it's definitely a typo. When I searched old chem community problems I found the same discussion.
- Wed Mar 07, 2018 9:08 am
- Forum: Student Social/Study Group
- Topic: Test 3
- Replies: 3
- Views: 646
Re: Test 3
I would expect everything before problem 15.45 to be covered.
- Tue Feb 27, 2018 5:38 pm
- Forum: Zero Order Reactions
- Topic: K0 vs K1
- Replies: 2
- Views: 590
Re: K0 vs K1
Can you clarify your question? I am not sure what is meant by k0. For zero order reactions, Rate = k so k is still present.
- Tue Feb 27, 2018 5:36 pm
- Forum: General Rate Laws
- Topic: 15.17
- Replies: 4
- Views: 650
Re: 15.17
Johann is right but to be clearer, first order in B means that the rate is proportional to 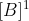 , second order in B means that the rate is proportional to
, second order in B means that the rate is proportional to 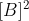 . and third order in B means that the rate is proportional to
. and third order in B means that the rate is proportional to 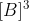 .
.
- Tue Feb 27, 2018 5:33 pm
- Forum: First Order Reactions
- Topic: 15.105
- Replies: 1
- Views: 288
15.105
To prepare a 15-kg dog for surgery, 150 mg of the anesthetic phenobarbitol is administered intravenously. The reaction in which the anesthetic is metabolized (decomposed in the body) is rst order in phenobarbitol and has a half-life of 4.5 h. After about 2 h, the drug begins to lose its effect. Howe...
- Wed Feb 21, 2018 6:34 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: #15.19-rate constant
- Replies: 2
- Views: 551
Re: #15.19-rate constant
I dont think chem mod actually looked at your work, you're on the right track but you should be multiplying by 10^-12 instead of 10^-3 because the units are mmol^-4 so you would need to think about that factor of -4 when converting units.
- Mon Feb 19, 2018 7:32 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation states
- Replies: 2
- Views: 227
Re: Oxidation states
The oxidation state of any element in its most stable form is 0.
- Mon Feb 19, 2018 7:31 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Inert Electrode
- Replies: 2
- Views: 456
Re: Inert Electrode
An inert electrode is needed when the species in the electrode do not conduct electricity, so you would use one in every situation except when the electrode is made of metals that would conduct electricity like transition metals.
- Mon Feb 19, 2018 7:23 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Galvanic Cells vs Electrochemical Cells
- Replies: 4
- Views: 802
Re: Galvanic Cells vs Electrochemical Cells
From LibreTexts: "There are two types of electrochemical cells: galvanic cells and electrolytic cells. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (ΔG < 0) to generate electricity. This type of electrochemical cell is often called a voltaic cell after ...
- Sat Feb 17, 2018 9:14 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Is enthalpy a state function?
- Replies: 12
- Views: 2240
Re: Is enthalpy a state function?
Enthalpy is a state function.
- Thu Feb 15, 2018 4:30 pm
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: Homework Q14.117
- Replies: 2
- Views: 412
Re: Homework Q14.117
14.117 The body functions as a kind of fuel cell that uses oxygen from the air to oxidize glucose: C_{6}H_{12}O_{6}(aq)+6O_{2}(g)\rightarrow 6CO_{2}(g)+6H_{2}O(l) During normal activity, a person uses the equivalent of about 10 MJ of energy a day. Assume that this val...
- Wed Feb 14, 2018 1:08 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Using Kelvin [ENDORSED]
- Replies: 3
- Views: 471
Re: Using Kelvin [ENDORSED]
The formula sheet online uses 273.15 so I would stick with that.
- Wed Feb 14, 2018 1:06 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Reversible
- Replies: 4
- Views: 537
Re: Reversible
As well as what Adrian said, a process can have delta U = 0 even with a temperature change if the system returns to its original state, since U is a state function.
- Wed Feb 14, 2018 1:04 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Maximum Energy Able to Do Work [ENDORSED]
- Replies: 3
- Views: 566
Re: Maximum Energy Able to Do Work [ENDORSED]
Can someone clarify what types of work? Some sites say maximum reversible work and others say maximum nonexpansion work.
- Sat Feb 10, 2018 4:42 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: O2 Microstates
- Replies: 2
- Views: 760
O2 Microstates
Does someone know why O2 has one microstate (in context of S=Kb lnW) instead of 2? Wouldn't translational motion mean that there would be 2 states (each O2 molecule on the left/ right), or is it different because both O2 molecules are identical?
- Tue Feb 06, 2018 2:56 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Tendency of processes
- Replies: 2
- Views: 335
Re: Tendency of processes
Manasa pointed out that a reaction will be spontaneous at low Gibbs energy, but as a whole reactions will proceed until they are at equilibrium where ΔG=0. Since ΔG = ΔGº + RT lnQ and ΔGº= -RT lnK, ΔG=0 when Q=K. Essentially, the reaction tries to minimize its Gibbs free energy as it proceeds toward...
- Tue Feb 06, 2018 2:48 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Differing Equations Used For delta S with Change in Temperature
- Replies: 3
- Views: 632
Re: Differing Equations Used For delta S with Change in Temperature
S=nRln(T2/T1) and S=nRln(P1/P2) are for isothermal expansion. S=Cln(T2/T1) is for expansion where temperature changes, but the system is at either constant pressure or volume.
- Tue Feb 06, 2018 2:41 pm
- Forum: Balancing Redox Reactions
- Topic: Ozone oxidation number
- Replies: 2
- Views: 299
Ozone oxidation number
What is the oxidation number of ozone (O3)? I think it's zero but I don't know if this qualifies a stable form of oxygen. I'm asking in context of problem 14.5a.
- Mon Jan 29, 2018 8:27 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy [ENDORSED]
- Replies: 6
- Views: 1349
Re: Gibbs Free Energy [ENDORSED]
Can you further clarify your question?
- Mon Jan 29, 2018 8:24 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 9.13
- Replies: 3
- Views: 403
Re: 9.13
I can confirm that you get the correct answer from using 1 mol of gas. There are also a few other problems in the homework with this assumption.
- Mon Jan 29, 2018 8:22 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs free energy equations
- Replies: 4
- Views: 631
Re: Gibbs free energy equations
Sorry, is there any way you could clarify what the molar Gibbs free energy is further? I am still slightly confused. Thank you for your response!
- Mon Jan 22, 2018 10:22 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs free energy equations
- Replies: 4
- Views: 631
Gibbs free energy equations
Is there a difference between using standard molar Gibbs energies and standard Gibbs free energies of formation to calculate \Delta G^{\circ} in the equation \Delta G^{\circ} = \Sigma nG_{m}^{\circ}(products)- \Sigma nG_{m}^{\circ}(reactants) (or \Delta G^{\circ} = \Sigma nG_{f}^{\ci...
- Mon Jan 22, 2018 10:11 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Confusion about entropy formula
- Replies: 6
- Views: 611
Re: Confusion about entropy formula
Does this basically mean the reaction takes place at a constant temperature?
- Mon Jan 22, 2018 10:11 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Confusion about entropy formula
- Replies: 6
- Views: 611
Confusion about entropy formula
\Delta S= q_{rev} / T apparently only works for heat transferred reversibly. How do we know when the situation has heat transferred reversibly, and what exactly does this mean? Quite a few of the homeworks so far have used this equation but there is no specification on the method of heat transfer. ...
- Wed Jan 17, 2018 2:59 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Is there a way specific way to approach into seeing if open closed or isolated?
- Replies: 9
- Views: 891
Re: Is there a way specific way to approach into seeing if open closed or isolated?
To elaborate on what Ryan said, a closed system allows the movement of energy but not mass. An open system allows the movement of energy and mass.
- Wed Jan 17, 2018 2:51 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Homework 8.93b
- Replies: 1
- Views: 286
Homework 8.93b
(a) Calculate the work that must be done against the atmosphere for the expansion of the gaseous products in the combustion of 1.00 mol C6H6(l) at 25 C and 1.00 bar. (b) Using data in Appendix 2A, calculate the standard enthalpy of the reaction. (c) Calculate the change in internal energy, U , of th...
- Wed Jan 17, 2018 2:46 pm
- Forum: Phase Changes & Related Calculations
- Topic: 8.91
- Replies: 1
- Views: 164
8.91
Can someone show their work for problem 8.91 in the homework? I don't understand how to relate rate of time to the other side of the equation. I figured: rate of heat transfer x time = heat to melt the ice + heat to change the temperature of the water to 5ºC And that rate of heat transfer is connect...
- Thu Jan 11, 2018 1:37 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Most Stable Forms
- Replies: 4
- Views: 1071
Most Stable Forms
How do we know the most stable form of an element, such that standard enthalpy of formation would be zero for that element? For instance, I would assume the most stable form of water would be H2O(l) because it is found in most commonly in nature, but its enthalpy of formation is -285.83. Is the most...
- Wed Jan 10, 2018 3:21 pm
- Forum: Phase Changes & Related Calculations
- Topic: Energy and Phase Changes
- Replies: 5
- Views: 575
Re: Energy and Phase Changes
I agree with Justin and Tatiana. I found this description on Quora to summarize: "In ice, water molecules are very closely packed together; the intermolecular forces of attraction are particularly strong in solids, which means that the molecules have little to no freedom to move. When you heat ...
- Wed Jan 10, 2018 3:16 pm
- Forum: Calculating Work of Expansion
- Topic: Homework Question 8.3
- Replies: 4
- Views: 656
Re: Homework Question 8.3
I will attach my work to the post, hope it helps!
- Mon Jan 08, 2018 4:04 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: 8.3 Reversible Isothermal Expansion
- Replies: 3
- Views: 399
8.3 Reversible Isothermal Expansion
8.3 describes, "In an isothermal expansion, the pressure of the gas falls as it expands (by Boyle’s law); so, to achieve reversible expansion, the external pressure must be reduced in step with the change in volume so that at every stage the external pressure is the same as the pressure of the ...

