Search found 35 matches
- Thu Mar 14, 2019 5:39 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Concentration Cells
- Replies: 1
- Views: 279
Re: Concentration Cells
Concentration cells contain the same components but at differing concentrations. So, the anode and cathode are the same in terms of what they are, but not their concentrations. For example, in class Lavelle used 0.1 M of Ag+ and 1 M of Ag+
- Mon Mar 11, 2019 3:00 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Determining the Magnitude of K
- Replies: 3
- Views: 576
Re: Determining the Magnitude of K
In lector today (3/11) Dr. Llavelle briefly spoke about what determines the magnitude of K, can someone clarify what he meant by this? It was toward the end of the lecture so I wasn't able to write everything down. Thank you. Are you referring to capital K(equilibrium)? or lowercase k (rate constan...
- Mon Mar 11, 2019 2:51 pm
- Forum: First Order Reactions
- Topic: Half-life Clarification
- Replies: 5
- Views: 620
Re: Half-life Clarification
Yes, because the half life equations are derived from the integrated rate laws. And the integrated rate laws differ in terms of reaction order.
This should help!
This should help!
- Thu Mar 07, 2019 4:15 pm
- Forum: Zero Order Reactions
- Topic: Concentration independent of the rate
- Replies: 4
- Views: 556
Concentration independent of the rate
When the concentration of a reactant is independent of the rate does that mean it's zero order? And how would we be able to tell given a table of initial rates/concentrations?
- Wed Mar 06, 2019 6:50 pm
- Forum: Zero Order Reactions
- Topic: Slope = -k
- Replies: 3
- Views: 474
Re: Slope = -k
This kind of helps answer your question.
- Wed Mar 06, 2019 6:46 pm
- Forum: General Rate Laws
- Topic: rate laws
- Replies: 3
- Views: 346
Re: rate laws
I think the difference is just that the Differential Rate Law uses derivatives, but we want to make the analysis easier/"better" so to get rid of the derivatives we integrate. So, the Differential Rate Law expresses the rate of the reaction as a function of change in concentration over tim...
- Fri Mar 01, 2019 10:26 am
- Forum: Balancing Redox Reactions
- Topic: Strength of reducing agent
- Replies: 10
- Views: 1968
Re: Strength of reducing agent
Chase Yonamine 1J wrote:The smallest (more negative) Reduction potential is a stronger reducing agent
Why is that?
- Fri Mar 01, 2019 10:25 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Does anyone know if test 2 will be curved?
- Replies: 15
- Views: 3029
Re: Does anyone know if test 2 will be curved?
Most likely not, since the first test wasn't. I would not expect test 2 to be curved. :(
- Thu Feb 28, 2019 4:54 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Pt inert electrode
- Replies: 9
- Views: 1814
Re: Pt inert electrode
Would you add Pt(s) to both sides?
- Mon Feb 25, 2019 6:38 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Difference in values
- Replies: 2
- Views: 283
Re: Difference in values
E cell refers to electrode potential of a cell.
Eo cell refers to standard electrode potential where electrode potential is measured at 1 atmosphere pressure, 1 molar solution at 25° C.(aka standard conditions)
Eo cell refers to standard electrode potential where electrode potential is measured at 1 atmosphere pressure, 1 molar solution at 25° C.(aka standard conditions)
- Sun Feb 24, 2019 11:20 pm
- Forum: Balancing Redox Reactions
- Topic: Test #2
- Replies: 9
- Views: 933
Re: Test #2
Lavelle mentioned in lecture on Friday that Test 2 would cover Gibbs Free Energy up until what the lecture covered Friday.
- Thu Feb 21, 2019 7:46 pm
- Forum: Balancing Redox Reactions
- Topic: 6K.1 7th Edition
- Replies: 1
- Views: 240
6K.1 7th Edition
H+ + Cr2O72-+C2H5OH ----> Cr3+ + H2O
The oxidized reaction is C2H5OH ---> C2H4O
The solutions manual says C is oxidized from 2- to 1- but when I do the math I get from 2- to 1+ , what am I doing wrong?
The oxidized reaction is C2H5OH ---> C2H4O
The solutions manual says C is oxidized from 2- to 1- but when I do the math I get from 2- to 1+ , what am I doing wrong?
- Thu Feb 21, 2019 6:18 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation States
- Replies: 10
- Views: 1008
Re: Oxidation States
This may be of some help!!! It can also be found on the course website
- Mon Feb 11, 2019 9:23 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Irreversible Expansions and Work
- Replies: 2
- Views: 319
Re: Irreversible Expansions and Work
Also, take a look at these two diagrams uploaded on the class website!!
- Mon Feb 11, 2019 9:18 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Irreversible Expansions and Work
- Replies: 2
- Views: 319
Re: Irreversible Expansions and Work
You've got it backwards, reversible expansion does more work. And will always do more work, this is because they tend to push against more external pressure and are slow processes. Reversible processes are also ideal situations where no energy is wasted. Irreversible expansion is spontaneous/ occurs...
- Mon Feb 11, 2019 9:01 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: When to use C=5/2R or C=3/2R
- Replies: 7
- Views: 3094
Re: When to use C=5/2R or C=3/2R
404982241 wrote:use the 5/2 when pressure is constant.
use 3/2 when volume is constant.
a great example is problem 8.31 in the 6th edition
Would these only be used when working with an ideal gas?
- Mon Feb 11, 2019 8:58 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Midterm [ENDORSED]
- Replies: 49
- Views: 4942
Re: Midterm [ENDORSED]
I've never had Lavelle before and I was wondering whether the questions on midterms/finals are mostly math based/problem solving or are there conceptual questions asked as well? They're mostly math based/problem solving but there are still conceptual questions, they're just worth less points./A lot...
- Sun Feb 10, 2019 7:55 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Lecture notes 2/8/19
- Replies: 3
- Views: 370
Re: Lecture notes 2/8/19
thank you so much!!!
- Fri Feb 08, 2019 6:52 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Lecture notes 2/8/19
- Replies: 3
- Views: 370
Lecture notes 2/8/19
I missed lecture because I had a midterm at the time, would anyone be open to sharing their notes? Please and thanks. :)
- Thu Feb 07, 2019 8:14 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: q= -w
- Replies: 8
- Views: 721
q= -w
What exactly is meant by q=-w or -q=w ?
- Tue Feb 05, 2019 3:29 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Midterm [ENDORSED]
- Replies: 49
- Views: 4942
Re: Midterm [ENDORSED]
I've never had Lavelle before and I was wondering whether the questions on midterms/finals are mostly math based/problem solving or are there conceptual questions asked as well?
- Tue Feb 05, 2019 3:25 pm
- Forum: Calculating Work of Expansion
- Topic: Difference between L.atm and Joules
- Replies: 2
- Views: 1656
Re: Difference between L.atm and Joules
Yes, you'll always have to multiply the answer in 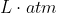 by 101.33 J to convert into J because as previously mentioned work is typically expressed in Joules. Also, my TA was uncertain as to whether this conversion will be on our equations sheet so that's something to keep in mind.
by 101.33 J to convert into J because as previously mentioned work is typically expressed in Joules. Also, my TA was uncertain as to whether this conversion will be on our equations sheet so that's something to keep in mind.
- Sun Feb 03, 2019 2:43 pm
- Forum: Calculating Work of Expansion
- Topic: Finding W Equations
- Replies: 1
- Views: 321
Re: Finding W Equations
Hi! So the reason we ended up back with w= -P\Delta V is because we were working with the DEFINITE integral which is basically V FINAL -V INITIAL which is just \Delta V Keep in mind though that this is only when pressure is constant. The derivative/integral was used for the reversible reaction examp...
- Wed Jan 30, 2019 6:16 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Thermo Test/Midterm
- Replies: 9
- Views: 789
Re: Thermo Test/Midterm
Most likely not, my TA also included drawing the lewis structures to account for bonds as one of the steps when calculating using bond enthalpies.
- Tue Jan 29, 2019 3:50 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: enthalpy
- Replies: 4
- Views: 569
Re: enthalpy
If you're a more visual person picture the image Lavelle presented in class of a mountain. There was a blue line and a red line, both lines started and ended in the same place. However, the path each line took was different! But because enthalpy is a state property that path doesn't matter because b...
- Wed Jan 23, 2019 5:17 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ICE boxes
- Replies: 5
- Views: 507
Re: ICE boxes
Yes! They can be, ICE tables help organize what you are given to solve equilibrium problems.
- Tue Jan 22, 2019 3:22 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Homework for Week 3
- Replies: 5
- Views: 498
Re: Homework for Week 3
Week 3 HW should just be problems concerning Acids and Bases.
- Tue Jan 22, 2019 3:17 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Ka and Kb
- Replies: 2
- Views: 278
Re: Ka and Kb
I'm certain that the Ka and Kb values will be given, we may be given pKa or pKb, but that works just as well because if we know the pKa or pKb value you can just take the antilog to find the Ka or Kb value.
- Fri Jan 18, 2019 7:07 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Test 1
- Replies: 6
- Views: 589
Re: Test 1
Will topics not discussed in lecture, but discussed in the reading be on the test?
- Fri Jan 18, 2019 7:04 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Weak Acid & Salt
- Replies: 4
- Views: 416
Weak Acid & Salt
I just want to make sure I understand something, for the example we did in class today: Calculate the pH of solution with 0.100 M HNO2 and 0.15 M KNO2, Ka= 4.3x10^-4 The chemical equation is: HNO2(aq) + H2O (l) (eq. arrows) NO2- + H3O+ because in the chemical equation (NO2+) + (K-) + (HNO2) (eq. arr...
- Thu Jan 17, 2019 1:54 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Test 1
- Replies: 6
- Views: 589
Test 1
How many questions should we expect on the first test Week 3?
- Mon Jan 14, 2019 5:56 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Acids and Bases
- Replies: 7
- Views: 882
Re: Acids and Bases
Then is there no way to determine whether an acid or base is strong by just looking at the compound itself? It's just memorization?
- Sun Jan 13, 2019 4:30 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Result of Only Adding One of the Reactants
- Replies: 4
- Views: 618
Re: Result of Only Adding One of the Reactants
Then, if you were to remove some reactant would the reaction shift left? Towards the reactants to compensate for the loss?
- Thu Jan 10, 2019 4:11 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Explaining Q<K and Q>K [ENDORSED]
- Replies: 7
- Views: 2317
Re: Explaining Q<K and Q>K [ENDORSED]
So, when Q<K it just means the reaction isn't at equilibrium, but before it right? Because there are more reactants than products, so in order to reach K the reaction will proceed forward. And when Q>K the reaction isn't at equilibrium, but after it? Because there are more products in the product/re...
- Tue Jan 08, 2019 4:01 pm
- Forum: Ideal Gases
- Topic: Memorization
- Replies: 12
- Views: 1286
Re: Memorization
I have a follow up question regarding memorization, I did not take 14A with Lavelle but my previous prof made us memorize some more obscure compounds. Could the same be said of Lavelle?

