Search found 30 matches
- Sat Mar 17, 2018 1:34 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Arrhenius Equation
- Replies: 3
- Views: 628
Re: Arrhenius Equation
Why do you use k2/k1?
- Sat Mar 17, 2018 1:28 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Lyndon Review Test, Question 7a [ENDORSED]
- Replies: 7
- Views: 951
Re: Lyndon Review Test, Question 7a [ENDORSED]
Where can I find Lyndon's review test?
- Sat Mar 17, 2018 1:27 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Negative activation energy?
- Replies: 2
- Views: 440
Re: Negative activation energy?
Where can I find this practice final?
- Sat Mar 17, 2018 1:26 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Activation Energy
- Replies: 5
- Views: 818
Re: Activation Energy
Since the reaction has multiple steps, there will be multiple transition states, which means multiple energy barriers.
- Sat Mar 17, 2018 1:24 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: catalyst
- Replies: 4
- Views: 657
Re: catalyst
Catalysts provide an alternate pathway for the reactants which requires less energy than without them.
- Sat Mar 17, 2018 1:19 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Transition State
- Replies: 3
- Views: 631
Re: Transition State
The transition state is the area between reactants and products where there is an energy barrier(activation energy) the must be met to complete the reaction. It is always the peak of the energy profile because all reactions have an energy barrier.
- Sun Mar 11, 2018 9:25 pm
- Forum: Zero Order Reactions
- Topic: Definition of Reaction Rate
- Replies: 4
- Views: 753
Re: Definition of Reaction Rate
It's given in units of M because we're studying the change in concentration, but there are questions using rate equations in the homework that involve mass of a given substance.
- Sun Mar 11, 2018 9:18 pm
- Forum: Zero Order Reactions
- Topic: Half-Life of Zero Order
- Replies: 3
- Views: 648
Re: Half-Life of Zero Order
Since Dr. Lavelle covered the derivation in class, you should assume it's fair game for the final just in case. It's on the equation sheet so you should be fine as long as you know what it is.
- Sun Mar 11, 2018 9:15 pm
- Forum: First Order Reactions
- Topic: Pseudo First Order Reaction
- Replies: 7
- Views: 1747
Re: Pseudo First Order Reaction
Expanding on this, what is a pseudo first order reaction?
- Sun Mar 04, 2018 10:08 pm
- Forum: General Rate Laws
- Topic: Rate equations
- Replies: 3
- Views: 550
Re: Rate equations
He hasn't asked for derivations on any of our tests so far, but knowing the derivation may help with applying the equation or answering conceptual questions.
- Sun Mar 04, 2018 10:05 pm
- Forum: General Rate Laws
- Topic: Instantaneous Rate
- Replies: 3
- Views: 517
Re: Instantaneous Rate
The instantaneous rate is the rate at one point one the reaction. More specifically, we use the instantaneous rate at time=0 so that no products have been formed and we can define the rate only with the concentration of the reactants.
- Sun Mar 04, 2018 10:02 pm
- Forum: General Rate Laws
- Topic: a value
- Replies: 3
- Views: 502
Re: a value
Are you talking about a as in - 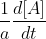 ? That a would be the molar coefficient of reactant A.
? That a would be the molar coefficient of reactant A.
- Sun Feb 25, 2018 10:01 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Pressure in calculating Q
- Replies: 4
- Views: 546
Re: Pressure in calculating Q
If you remember from 14A as long as we are studying something in a gaseous state we can use partial pressure or concentration to calculate Q since the pressure of gas will also affect the reaction direction.
- Sun Feb 25, 2018 9:54 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Nernst Equation to find pH
- Replies: 6
- Views: 10228
Re: Nernst Equation to find pH
Using the Nernst equation you can find the concentration of H+ ions which you can use to find pH. There was a homework problem that gave you pH and asked to find E, try looking at the solution for that if you're still confused.
- Sun Feb 25, 2018 9:50 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: anode vs cathode in non standard cell diagram
- Replies: 5
- Views: 741
Re: anode vs cathode in non standard cell diagram
The anode is usually on the left but if you're not sure you can just check which has the higher Eo value
- Sun Feb 11, 2018 11:50 pm
- Forum: Van't Hoff Equation
- Topic: Derivation
- Replies: 9
- Views: 1270
Re: Derivation
It's unlikely the derivations will appear on a test since we're given the equations, but as the above users have said it would be helpful to know them since understanding them would help you know how to apply them.
- Sun Feb 11, 2018 11:36 pm
- Forum: Van't Hoff Equation
- Topic: Finding K
- Replies: 5
- Views: 1039
Re: Finding K
Dr. Lavelle has mentioned in lecture that we won't need to to ICE tables
- Sun Feb 11, 2018 11:35 pm
- Forum: Van't Hoff Equation
- Topic: Reaction Coefficient, Q
- Replies: 2
- Views: 560
Re: Reaction Coefficient, Q
The lowercase letters a, b, c, and d all refer to the molar coefficients of each part of the reaction, and the upper case letters A, B, C, and D refer to the components of the reaction, with C and D as products and A and B as reactants.
- Sun Feb 04, 2018 2:56 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Residual Entropy
- Replies: 4
- Views: 607
Re: Residual Entropy
Is residual entropy related to how there is a standard entropy of formation for gas molecules like O2 that are already in their standard state?
- Sun Feb 04, 2018 2:53 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Perfect Crystal
- Replies: 9
- Views: 1512
Re: Perfect Crystal
Can someone explain why entropy of a "perfect crystal" is 0 (S=0)? At 0K there is no movement of atoms so there is only one possible state for each molecule in a crystal, so it is perfectly ordered and we can know the exact position of everything. Since there is only one possible state, d...
- Sun Feb 04, 2018 2:45 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Third Law
- Replies: 3
- Views: 428
Re: Third Law
Because there is no movement at 0K, there is only one possible state (so w=1), which makes entropy equal to 0.
- Sun Jan 28, 2018 10:55 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: About entropy and gibbs free energy
- Replies: 2
- Views: 341
Re: About entropy and gibbs free energy
To piggyback on the post above, the total ∆G of the two reactions combined must still be negative, so the spontaneous reaction must release more free energy than the non-spontaneous reaction uses.
- Sun Jan 28, 2018 10:25 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Boltzmann Equation Clarification
- Replies: 3
- Views: 337
Re: Boltzmann Equation Clarification
Can someone explain how it depends on temperature? Since T isn't part of the equation, are there different values of Kb depending on the temperature?
- Sun Jan 28, 2018 10:15 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Positional disorder?
- Replies: 2
- Views: 338
Re: Positional disorder?
Potential disorder is another way of saying the amount of potential different states of a system, so it is the same as degeneracy. This shouldn't be important though since Dr. Lavelle wants to avoid using the term disorder altogether.
- Fri Jan 19, 2018 12:00 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Specific Heat Capacity
- Replies: 5
- Views: 2054
Re: Specific Heat Capacity
I think Dr. Lavelle said in lecture on Wednesday that the solution is so dilute that that salt(in the case of acid-base reactions) has very little impact on the specific heat capacity and you can just use the capacity of the solvent.
- Thu Jan 18, 2018 11:52 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Molar heat capacity
- Replies: 3
- Views: 351
Re: Molar heat capacity
Using the ideal gas equation, 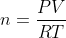 , so the number of moles of a certain gas can change as pressure or volume change.
, so the number of moles of a certain gas can change as pressure or volume change.
- Thu Jan 18, 2018 11:47 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: State function in relation to heat
- Replies: 3
- Views: 462
Re: State function in relation to heat
Adding to the other posts, when we calculate the change in internal energy of a system, ∆U = Q + W, Q represents the energy that the system either loses or gains as heat, not the heat the system possesses.
- Thu Jan 11, 2018 3:23 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Enthalpy of sublimation
- Replies: 3
- Views: 793
Re: Enthalpy of sublimation
Another way to think about this is to remember that enthalpy is a state function, so the path taken doesn't matter. Since sublimation is changing directly from a solid to a vapor, the beginning and end points are the same as a a substance that goes through fusion and then vaporization, so the ∆H of ...
- Thu Jan 11, 2018 3:20 pm
- Forum: Phase Changes & Related Calculations
- Topic: Ideal Gas
- Replies: 5
- Views: 447
Re: Ideal Gas
If something behaves like an ideal gas, it means it follows the ideal gas law PV=nRT.
- Mon Jan 08, 2018 6:01 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Having the textbook in discussion
- Replies: 2
- Views: 332
Having the textbook in discussion
Do we need to bring a physical copy of the textbook to discussion sections, or can we use a pdf on our laptops?

