Search found 30 matches
- Wed Mar 14, 2018 10:37 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: adiabatic process
- Replies: 5
- Views: 857
Re: adiabatic process
Both open and closed systems can have adiabatic processes since heat is another form of energy (first law) and energy can be exchanged through both kinds of systems.
- Wed Mar 14, 2018 10:31 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Calculating Gibbs Free Energy
- Replies: 4
- Views: 664
Calculating Gibbs Free Energy
How do we know when to do the difference of sums vs when to use the equation 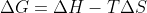 to solve for gibbs free energy?
to solve for gibbs free energy?
- Wed Mar 14, 2018 10:29 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.99 [ENDORSED]
- Replies: 1
- Views: 367
8.99 [ENDORSED]
Does the solutions manual use the enthalpy of formation value for Zn2+ in replacement for ZnCl because the Cl is just an ion that has a 2- charge? if so, would this also be true when looking for the enthalpy of formation values for all elements attached to an ion?
- Wed Mar 14, 2018 10:25 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.101 [ENDORSED]
- Replies: 2
- Views: 360
8.101 [ENDORSED]
For this problem, why is 2SO2 the limiting reactant when O2 takes the same amount of moles while its coefficient is 1? Looking at how the solutions manual determined the limiting reactant for the previous problem, shouldn't O2 be considered the limiting reactant here?
- Wed Mar 14, 2018 10:22 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.57
- Replies: 1
- Views: 270
8.57
How do we know when we are supposed to write out the equations and use a Hess' law approach to solve for the reaction enthalpy? For this problem, i thought we were supposed to use the difference of sums approach because i didn't initially know how to write the combustion reactions
- Wed Mar 14, 2018 10:18 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.67 [ENDORSED]
- Replies: 1
- Views: 230
8.67 [ENDORSED]
For this problem, how do you know that adding the bond enthalpies of a reaction will give you the change in enthalpy for the production of water in the gas phase? In general, how would you determine what state the element will be formed in?
- Tue Feb 27, 2018 9:38 pm
- Forum: First Order Reactions
- Topic: Difference between 1st and 2nd order reactions
- Replies: 3
- Views: 21725
Re: Difference between 1st and 2nd order reactions
Also, in a first order reaction, the rate of the reaction is doubled while in a second order reaction, the rate of the reaction is quadrupled.
- Tue Feb 27, 2018 9:32 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell diagram order
- Replies: 4
- Views: 487
Re: Cell diagram order
The written cell diagram typically mirrors the set up of the battery in that the anode(oxi) is on the left while the cathode(red) is on the right side.
- Tue Feb 27, 2018 9:23 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Unique Average Rate vs. Average Rate
- Replies: 3
- Views: 591
Re: Unique Average Rate vs. Average Rate
The unique rate relates to the production/consumption of one mole of a substance substance (you get this by dividing by its stoichiometric coefficient) while average rate is the overall rate for the whole reaction over a period of time.
- Mon Feb 19, 2018 5:43 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 14.37
- Replies: 2
- Views: 470
14.37
I understand how to plug all the values into the NERNST equation after I write the half reactions, but I'm confused as to where the solutions manual is getting 0.025693V/n. I thought we were supposed to use either 2.303RT/nF or 0.0592/n?
- Sun Feb 18, 2018 12:19 am
- Forum: Balancing Redox Reactions
- Topic: Test 2
- Replies: 5
- Views: 665
Test 2
On test 2, will we be given a chart of all the half reactions and their corresponding 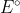 values such as the one found in Appendix 2B, or will we have to write our own half reactions and just be given the
values such as the one found in Appendix 2B, or will we have to write our own half reactions and just be given the 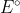 ?
?
- Sat Feb 17, 2018 11:31 pm
- Forum: Balancing Redox Reactions
- Topic: Sign of E when anode rxn reverses
- Replies: 2
- Views: 348
Sign of E when anode rxn reverses
As i am doing the homework questions (specifically #11-15) I noticed the solutions manual reverses the anode reaction in every problem but only sometimes changes the sign of E^{\circ} . I am confused on why the E^{\circ} sign is not being switched every time the anode reaction is reversed? How do we...
- Mon Feb 12, 2018 4:53 pm
- Forum: Calculating Work of Expansion
- Topic: Derivations
- Replies: 2
- Views: 405
Re: Derivations
In lecture, Dr. Lavelle said we will not explicitly be asked "derive the equation for _____", but for some problems we will need to know the different variations of the original equation in order to solve the problem. So to answer your last question, yes, we have to be able to apply the co...
- Mon Feb 12, 2018 4:44 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Units for enthalpy
- Replies: 2
- Views: 427
Re: Units for enthalpy
It's safe to just look at what you are given in the question (if enthalpy is in kj/mol in the question then your answer should be in kj/mol, etc.) but you can also assume that you can put just kj for your answer if the question implies that you're only talking about one mole of a substance.
- Mon Feb 12, 2018 4:04 pm
- Forum: Calculating Work of Expansion
- Topic: q and temp increase
- Replies: 2
- Views: 384
Re: q and temp increase
a negative q does not necessarily mean that the temperature will decrease as shown by the heat curve: The diagonal lines indicate an increase/decrease in temperature as a result of the change in energy, the flat parts of the graph show that energy is being added/removed however the temperature does ...
- Wed Feb 07, 2018 10:28 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: formula
- Replies: 3
- Views: 395
Re: formula
This formula is useful as it represents what the second law of thermodynamics is, but it is mostly used as the equation from which we derive the other entropy equations we use more frequently for calculations.
- Wed Feb 07, 2018 10:19 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Delta G
- Replies: 3
- Views: 461
Re: Delta G
\Delta G^{\circ} is the change in free energy under standard conditions (25celcius and 1atm) while \Delta G is the change in free energy under varying temp and pressure conditions. If you're calculating \Delta G^{\circ} , your \Delta H^{\circ} and \Delta S^{\circ} also need to be under standard con...
- Wed Feb 07, 2018 10:11 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs free energy and work
- Replies: 3
- Views: 452
Gibbs free energy and work
I was a little confused in lecture when prof Lavelle said that the maximum work done by a process represents the free energy for that system, can somebody explain this to me conceptually? Also, what does the temp and pressure have to do with this idea?
- Mon Jan 29, 2018 10:24 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: R value in entropy equation [ENDORSED]
- Replies: 2
- Views: 494
R value in entropy equation [ENDORSED]
When solving for entropy with changes in volume and temperature, how do we know when we have to multiply R by 3/2 or 5/2 vs when we just use the 8.314 in our equation?
- Mon Jan 29, 2018 10:15 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Temperature units for Entropy [ENDORSED]
- Replies: 2
- Views: 441
Temperature units for Entropy [ENDORSED]
When solving for entropy when there is a change in temperature while Volume is constant, does it matter if we calculate the T2/T1 in Kelvin or Celcius? If so, which one are we expected to be using?
- Mon Jan 29, 2018 10:09 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Relationship between Enthalpy and Entropy [ENDORSED]
- Replies: 1
- Views: 304
Relationship between Enthalpy and Entropy [ENDORSED]
I'm a little confused on why there is a negative relationship between  and
and 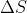 ? If
? If  wouldn't that make
wouldn't that make 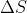 a negative number since
a negative number since 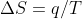 ?
?
- Fri Jan 26, 2018 4:27 pm
- Forum: Phase Changes & Related Calculations
- Topic: When to use Kelvin or Celsius
- Replies: 10
- Views: 6897
Re: When to use Kelvin or Celsius
when you're doing a problem that calls for the change in temperature it doesn't matter what units you use because the difference in temp will be the same no matter what units you're in, but the problem should specify what units if they are needed.
- Fri Jan 26, 2018 4:21 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Irreversible vs. Reversible [ENDORSED]
- Replies: 10
- Views: 1581
Re: Irreversible vs. Reversible [ENDORSED]
how does the equation w=-nRTlnV2/V1 account for the changing pressure and more work being done as opposed to the equation for the irreversible pathway?
- Fri Jan 26, 2018 4:06 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Formula confusion [ENDORSED]
- Replies: 2
- Views: 191
Re: Formula confusion [ENDORSED]
S = Kb lnW is the standard equation for finding entropy while S = Kb ln2 is simply the standard equation with the degeneracy value plugged in.
- Fri Jan 19, 2018 10:08 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Enthalpy at constant pressure and volume [ENDORSED]
- Replies: 3
- Views: 311
Re: Enthalpy at constant pressure and volume [ENDORSED]
I had the same question as i was doing the homework questions but i think that is something he will go over in lecture on Friday.
- Fri Jan 19, 2018 10:03 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Internal Energy/Open System
- Replies: 4
- Views: 596
Re: Internal Energy/Open System
You can also change it by adding or removing different amounts of the substances in an open system.
- Fri Jan 19, 2018 10:01 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 8.29
- Replies: 4
- Views: 290
Re: 8.29
Yes, the larger the molecule, the more bonds there are that have to be accounted for when it is being heated.
- Fri Jan 12, 2018 9:18 pm
- Forum: Phase Changes & Related Calculations
- Topic: Boiling water vs. steam
- Replies: 6
- Views: 1098
Re: Boiling water vs. steam
The condensation of the water vapor when it touches your skin releases large amounts of energy(heat) that causes the burns.
- Fri Jan 12, 2018 9:07 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: 8.1 part c [ENDORSED]
- Replies: 8
- Views: 1168
Re: 8.1 part c [ENDORSED]
By definition, an isolated system can't exchange matter or energy with its surroundings and a calorimeter does not allow for this exchange.
- Fri Jan 12, 2018 9:01 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Problem 8.31
- Replies: 3
- Views: 335
Re: Problem 8.31
Will these Cpm and Cvm values always be given for the problem?

