Search found 30 matches
- Sun Mar 18, 2018 1:54 am
- Forum: Administrative Questions and Class Announcements
- Topic: Lavelle: Professor by day, wizard by night
- Replies: 6
- Views: 1536
Re: Lavelle: Professor by day, wizard by night
Well... this is definitely what I needed to lighten up before the final lol
- Sat Mar 17, 2018 8:53 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Inert Electrodes
- Replies: 1
- Views: 372
Re: Inert Electrodes
You need to add an inert electrode, such as platinum (Pt), when there are no other conducive metals in a half reaction in order to allow current to flow through the cell. You'd just need to add the electrode to the side that doesn't have a solid metal e.g. Zn(s) | Zn 2+ (aq) || Sn 4+ (aq),Sn 2+ (aq)...
- Sat Mar 17, 2018 8:42 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Practice Midterm W18 #3A
- Replies: 3
- Views: 571
Re: Practice Midterm W18 #3A
Essentially, you would solve for the entropy at each individual step then add the totals up; this can be done because entropy is a state function. Your first step would to be to calculate the change in entropy due to the change in volume of helium gas. Step two would be to calculate the entropy chan...
- Sat Mar 17, 2018 6:03 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Organic Chem
- Replies: 2
- Views: 535
Re: Organic Chem
I'm pretty sure Professor Lavelle said that we will need to know the functional groups we went over in lecture. We also talked about SN2 substitution reactions and went over those on the practice final, so I don't think it would hurt to review that as well.
- Sat Mar 17, 2018 5:57 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Cp & Cv?
- Replies: 3
- Views: 718
Re: Cp & Cv?
No,  does not have to equal zero in a bomb calorimeter, meaning pressure is not a constant.
does not have to equal zero in a bomb calorimeter, meaning pressure is not a constant.
- Sat Mar 17, 2018 1:21 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Slow Step Determining Overall Rate
- Replies: 3
- Views: 1115
Slow Step Determining Overall Rate
Under what conditions does the rate law of the slow step determine the overall rate law of the reaction in a reaction mechanism? Is it when the slow step is the first step of the reaction mechanism?
- Mon Mar 12, 2018 7:38 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589273
Re: Post All Chemistry Jokes Here
Q: What is the name of 007's Eskimo cousin?
A: Polar Bond.
A: Polar Bond.
- Mon Mar 12, 2018 12:23 pm
- Forum: Calculating Work of Expansion
- Topic: Practice Problem
- Replies: 1
- Views: 328
Re: Practice Problem
I think you're correct Michelle and agree with your conclusion; the value should be positive because of \Delta U=q+w . Additionally, part b should be work done on the system. Perhaps a typo in the question? Typo could make sense given the 982 kJ - 492 kJ = +490 kJ, not 491... I think you're correct,...
- Mon Mar 12, 2018 12:10 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Catalyst Example from lecture 3/12
- Replies: 3
- Views: 566
Re: Catalyst Example from lecture 3/12
I'm pretty sure the catalyst was NO (g).
- Sat Mar 03, 2018 6:39 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Rate Constant Units
- Replies: 9
- Views: 1338
Re: Rate Constant Units
You can use the rate law rate=k[A]^{n} and plug in the units for each variable and simplify. For example, for zero order reactions: \frac{mol A}{L\cdot s} = k[A]^{0} \frac{mol A}{L\cdot s} = k For first order: \frac{mol A}{L\cdot s} = k[A]^{1} \frac{mol A}{L\cdot s} = k(\frac{mol A}{L}) \fra...
- Sat Mar 03, 2018 5:58 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589273
Re: Post All Chemistry Jokes Here
Q: Anyone know any jokes about sodium?
A: Na
A: Na
- Sat Mar 03, 2018 5:33 pm
- Forum: General Rate Laws
- Topic: 15.27
- Replies: 2
- Views: 462
Re: 15.27
Using the given half-life, we can use the formula  to calculate the k value. From there, we can use the formula
to calculate the k value. From there, we can use the formula 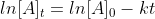 to calculate time. [A]t is the concentration remaining after time t.
to calculate time. [A]t is the concentration remaining after time t.
- Sat Feb 24, 2018 8:37 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation state
- Replies: 5
- Views: 704
Re: Oxidation state
In short, yes. The oxidation number represents the number of electrons that an element can lose (if negative) or gain (if positive), relative to the electronegativity of any other element is in combination with. Because O 2 and H 2 have the same electronegativity, respectively, their oxidation numbe...
- Sat Feb 24, 2018 8:25 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: When do you need to include Pt? [ENDORSED]
- Replies: 5
- Views: 703
Re: When do you need to include Pt? [ENDORSED]
No you do not always need to include Pt in your cell diagram. Platinum is used when there are no other inert metals in the reaction in order to allow current to flow through the cell. Most the time, you see Pt(s) being used when you are dealing with a cell that has a half-reaction that does not have...
- Sat Feb 24, 2018 8:20 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: n
- Replies: 8
- Views: 902
Re: n
I believe in lecture Friday, Professor Lavelle also used 'n' to represent the order of the reactant in the "rate law" ( 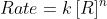 ) if that is what you are referring to when you say kinetics.
) if that is what you are referring to when you say kinetics.
- Sat Feb 10, 2018 7:36 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Arithmetic in 11.19c
- Replies: 2
- Views: 476
Re: Arithmetic in 11.19c
http://i1380.photobucket.com/albums/ah165/andresreynoso/chemcomm_zps6ilqogyd.jpg
Hopefully this helps!
*Edit: Photobucket doesn't allow third party hosting, so I just submitted the direct link.
Hopefully this helps!
*Edit: Photobucket doesn't allow third party hosting, so I just submitted the direct link.
- Sat Feb 10, 2018 6:54 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 11.111
- Replies: 2
- Views: 348
Re: 11.111
Because the question asks for the standard Gibbs free energy of the second reaction, we assume that the temperature the reaction occurred at is 25°C since that is the accepted temperature for standard conditions.
- Sat Feb 10, 2018 6:34 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Explain Figure 9.25...
- Replies: 1
- Views: 290
Re: Explain Figure 9.25...
The graph is essentially showing us the relationship between temperature and the (molar) Gibbs free energy of a substance at a given pressure and how they both correlate to what phase a certain substance in in. It shows us that at low temperatures, the Gibbs free energy is lowest for solids. Because...
- Sat Feb 03, 2018 8:09 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: G at minimum
- Replies: 6
- Views: 581
Re: G at minimum
Gibbs free energy is essentially a measure of how much energy we can get from a system. So if the free energy is at a minimum, then the reaction is at equilibrium and no more change in energy can be done in the system. If anyone would like to add, please feel free.
- Sat Feb 03, 2018 7:47 pm
- Forum: Calculating Work of Expansion
- Topic: Confused about work formulas and specific heats
- Replies: 2
- Views: 451
Re: Confused about work formulas and specific heats
I'm pretty sure Cv and Cp are only to be used when the conditions dictate that the system is under constant volume or pressure, respectively, which is why it is often used when temperature is changing. For example, using Cp for a system where pressure is changing wouldn't work since pressure must be...
- Sat Feb 03, 2018 7:34 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Example from Wednesday's Lecture
- Replies: 3
- Views: 363
Example from Wednesday's Lecture
Professor Lavelle did an example on Wednesday where he asked at what temperature is Br(l)\rightarrow Br(g) spontaneous at 1 atm given \Delta H^{o}= 31.0 kJ/mol and \Delta S^{o}= 93.0 J/Kmol . He said that we were looking to find the temperature when \Delta G^{o}= 0 and said that neit...
- Sat Jan 27, 2018 5:32 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 9.7 and 9.11 formula differences
- Replies: 4
- Views: 574
Re: 9.7 and 9.11 formula differences
The difference lies in what the question is asking. For 9.7 part a, the question asks for the entropy change at a constant pressure with a change in temperature. For this we use the known equation \Delta S=nC_{p}ln\frac{T_{2}}{T_{1}} where C p is the specific heat of a gas at constant pressure. For ...
- Sat Jan 27, 2018 5:04 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bondy Enthalpy Accuracy
- Replies: 3
- Views: 643
Re: Bondy Enthalpy Accuracy
Using bond enthalpies is the least accurate method to calculate standard reaction enthalpies because bond enthalpies are averages of the dissociation enthalpies of bonds from several different molecules. Essentially, they are the least accurate because it is an average, where other methods are more ...
- Sat Jan 27, 2018 4:41 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: isothermal system
- Replies: 4
- Views: 420
Re: isothermal system
Yes, when a system is isothermal it means that there is no temperature change in the system and thus the temperature would be constant. Only for ideal gases does this mean that the change in internal energy is 0 because, for ideal gases, \Delta U = \frac{3}{2}nR\Delta T and \Delta T = 0 (Anything mu...
- Sat Jan 20, 2018 4:26 pm
- Forum: Phase Changes & Related Calculations
- Topic: HW #1 PART E
- Replies: 3
- Views: 524
Re: HW #1 PART E
Mercury in a thermometer is a closed system because only energy can be exchanged with its surroundings. Matter cannot be exchanged within the system. Here is a more detailed example for the thermometer's case: A thermometer is placed under someone's tongue. The heat (energy) from their body (surroun...
- Sat Jan 20, 2018 4:22 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Specific Heat Capacity
- Replies: 3
- Views: 435
Re: Specific Heat Capacity
Yes it does stand for Kelvin. It is equivalent to degrees Celsius because specific heat equations usually involve temperature change . The magnitude for temperature change is the same for Kelvin and Celsius; so both units are interchangeable for specific heat capacity problems depending on which tem...
- Sat Jan 20, 2018 4:03 pm
- Forum: Phase Changes & Related Calculations
- Topic: Temperature [ENDORSED]
- Replies: 2
- Views: 178
Re: Temperature [ENDORSED]
For temperature changes , it doesn't matter if it is degrees Celsius or degrees Kelvin because the value of the difference would be the same (e.g. 1 C heats to 5 C = +4 C; 274.15 K heats to 278.15 K = +4 K). When using a formula that doesn't involve temperature change, where the other units indicate...
- Sat Jan 13, 2018 5:42 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bond Enthalpy vs. Bond Dissociation Enthalpy
- Replies: 3
- Views: 633
Bond Enthalpy vs. Bond Dissociation Enthalpy
What is the difference between these two terms? I do not think bond dissociation enthalpy has not been discussed in class but I came across the term reading online and am curious.
- Sat Jan 13, 2018 5:26 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Question 8.41
- Replies: 2
- Views: 214
Re: Question 8.41
There is a negative sign in front of the value for heat (water) because it is the heat lost from the initial sample of water that is being gained by the water of the ice cube. The negative sign indicates the loss of that heat.
Edit: I agree with Jessica above.
Edit: I agree with Jessica above.
- Sat Jan 13, 2018 11:07 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Heat Capacity
- Replies: 3
- Views: 386
Re: Heat Capacity
I think that, for the scope of this class, the specific heat capacity of a substance will always be provided, given that the specific heat of a substance is determined through experimentation.

