Search found 39 matches
- Fri Mar 16, 2018 12:19 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 9.103
- Replies: 1
- Views: 371
Re: 9.103
The values are in Appendix 2A, but you have to scroll down to the organic compounds section. It starts on page A15.
- Thu Mar 15, 2018 3:27 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Test 2 Question 8b
- Replies: 9
- Views: 1493
Re: Test 2 Question 8b
You can use both partial pressure and concentration in the equilibrium expression as long as the pressure is in bars and the concentration is in molarity.
- Thu Mar 15, 2018 3:20 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Coefficients in Rate Law
- Replies: 2
- Views: 428
Re: Coefficients in Rate Law
You'll be doing this when dealing with reaction mechanisms. Because an elementary reaction shows how the step of a reaction occurs, we can write the rate law of elementary reactions (not the overall reaction) with each exponent being the number of molecules of a particular type participating in that...
- Thu Mar 15, 2018 3:13 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: emf
- Replies: 3
- Views: 513
Re: emf
emf is another way to mean the cell potential. They are the same thing.
- Tue Mar 06, 2018 10:20 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: 15.19a
- Replies: 2
- Views: 410
Re: 15.19a
To find the order of [B] you want to compare reaction 1 and 3 because the concentrations of A and C stay the same. So we have \frac{Rate 3}{Rate 1} = \frac{50.8}{8.7} = \frac{k(1.25)^{N}(3.02)^{M}(1.25)^{L}}{k(1.25)^{N}(1.25)^{M}(1.25)^{L}} 5.84 = 2.41...
- Mon Mar 05, 2018 11:26 pm
- Forum: General Rate Laws
- Topic: 15.17
- Replies: 4
- Views: 585
Re: 15.17
The rate doesn't depend on the concentration of C, which means it is independent, because the reaction is zero order with respect to C. If you compare experiments 1 and 4 (where the concentrations of A and B stay the same), the initial concentration of C changes but the initial rate doesn't change.
- Mon Mar 05, 2018 11:23 pm
- Forum: Second Order Reactions
- Topic: 15.19
- Replies: 2
- Views: 565
Re: 15.19
To find the order of [B] you want to compare reaction 1 and 3 because the concentrations of A and C stay the same. So we have \frac{Rate 3}{Rate 1} = \frac{50.8}{8.7} = \frac{k(1.25)^{N}(3.02)^{M}(1.25)^{L}}{k(1.25)^{N}(1.25)^{M}(1.25)^{L}} 5.84 = 2.41...
- Mon Mar 05, 2018 11:21 pm
- Forum: General Rate Laws
- Topic: 15.17
- Replies: 3
- Views: 474
Re: 15.17
If you compare experiments 1 and 4 (where the concentrations of A and B stay the same), you see that the initial concentration of C changes but the initial rate doesn't change. Therefore, the reaction is zero order with respect to C.
- Sat Mar 03, 2018 3:49 pm
- Forum: General Rate Laws
- Topic: 15.3 partb ?
- Replies: 3
- Views: 509
Re: 15.3 partb ?
That isn't the rate law but the unique rate of the reaction. In part A, you should have calculated the rate of reaction of NO 2 . In general for all reactions, aA -> bB + cC rate = \frac{1}{a}\frac{d[A]}{dt} = \frac{1}{b}\frac{d[B]}{dt} = \frac{1}{c}\frac{d[C]}{dt} The reactants and products in a re...
- Sat Mar 03, 2018 3:35 pm
- Forum: First Order Reactions
- Topic: Half Lives
- Replies: 2
- Views: 414
Re: Half Lives
I think it is because the half-lives of zero order and second order reactions depend on the concentration, so it is constantly changing as the concentration changes. In contrast, first order reaction half lives don't depend on concentration and as such are always constant, which makes it easier to u...
- Sat Mar 03, 2018 3:27 pm
- Forum: General Rate Laws
- Topic: 15.19 part a
- Replies: 1
- Views: 264
Re: 15.19 part a
To find the order of [B] you want to compare reaction 1 and 3 because the concentrations of A and C stay the same. So we have \frac{Rate 3}{Rate 1} = \frac{50.8}{8.7} = \frac{k(1.25)^{N}(3.02)^{M}(1.25)^{L}}{k(1.25)^{N}(1.25)^{M}(1.25)^{L}} 5.84 = 2.41...
- Sat Mar 03, 2018 1:37 pm
- Forum: General Rate Laws
- Topic: Unique Rate [ENDORSED]
- Replies: 5
- Views: 1035
Re: Unique Rate [ENDORSED]
In addition, when you calculate the unique rate, it is the same for all the reactants and products involved in the reaction.
- Fri Mar 02, 2018 3:13 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: E = E^o - (RT/nF) ln Q [ENDORSED]
- Replies: 4
- Views: 661
Re: E = E^o - (RT/nF) ln Q [ENDORSED]
R = 8.314 J / (mol x K) and T is in K.
- Fri Feb 23, 2018 4:23 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Reaction Order [ENDORSED]
- Replies: 2
- Views: 458
Re: Reaction Order [ENDORSED]
If the reaction depends on the concentrations of two or more reactants, you could say it is first order with respect to the reactant. So in the example in class today, it is first order with respect to NH 4 + (or in NH 4 + ) and first order in NO 2 - . And the overall order is the sum so it is a sec...
- Fri Feb 23, 2018 4:15 pm
- Forum: General Rate Laws
- Topic: Rate Constant K [ENDORSED]
- Replies: 4
- Views: 659
Re: Rate Constant K [ENDORSED]
The value of the rate constant K varies depending on the temperature and activation energy which can be seen through the Arrhenius equation. For example, raising the temperature of the reaction will increase the value of the rate constant K and therefore increase the speed at which the reaction is o...
- Fri Feb 23, 2018 4:11 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Determining N
- Replies: 4
- Views: 629
Re: Determining N
It doesn't matter. In the example in class, with rate 2 on top we got 2 = 2n so n = 1. If we do rate 1/rate 2, we get 1/2 = (1/2)n so n will still equal one.
- Thu Feb 15, 2018 8:16 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Adiabatic Reaction
- Replies: 2
- Views: 428
Re: Adiabatic Reaction
Adiabatic means there is no heat transfer so q = 0. From 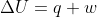 we get that
we get that 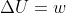 .
.
- Thu Feb 15, 2018 8:05 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Chem Community Posts
- Replies: 5
- Views: 882
Re: Chem Community Posts
My TA said that posts are counted weekly, and you won't be able to make up points if you forget to post 3 times during a week.
- Wed Feb 14, 2018 4:13 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Practice Midterm #3b Equation
- Replies: 3
- Views: 507
Re: Practice Midterm #3b Equation
V = 11 L is given. n equals the sum of the He and Kr moles. It should be around 3.74 moles. R is the gas constant. T is the final temperature in K which is 75.0 + 273.15 = 348.15 K. Plug in and solve for P.
- Wed Feb 14, 2018 2:25 am
- Forum: Phase Changes & Related Calculations
- Topic: Specific heat of water or ice?
- Replies: 6
- Views: 1135
Re: Specific heat of water or ice?
You would use the specific heat of ice when it is in the ice phase. For example, say you want to find the q added to the system to get ice at -10 degrees celsius to water at +10 degrees celsius. You would find q needed to raise temperature from -10 to 0 degrees using specific heat of ice in the equa...
- Wed Feb 14, 2018 2:10 am
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Test 1 Question 3b
- Replies: 2
- Views: 418
Re: Test 1 Question 3b
Since the system is isothermal, the change in internal energy is 0. So q+w= 0, and q=-w. Since the balloon is expanding its volume against a changing pressure, work is being done. The energy lost during work is gained as heat, because the internal energy must equal 0.
- Wed Feb 14, 2018 2:06 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Practice Midterm #3b Equation
- Replies: 3
- Views: 507
Re: Practice Midterm #3b Equation
PV = nRT
- Wed Feb 14, 2018 2:00 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Test 1 3a
- Replies: 1
- Views: 263
Re: Test 1 3a
Heat is transferred because it is needed for a phase change from liquid to gas.
- Sat Feb 10, 2018 3:11 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: When to use + sign
- Replies: 11
- Views: 1072
Re: When to use + sign
On page 320 of the textbook it says, "A Note on Good Practice: Note the sign on the answer; always show the sign explicitly for the change in a quantity, even if it is positive." You wouldn't get marked wrong if you didn't have the positive sign though.
- Sat Feb 10, 2018 2:58 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: When to use + sign
- Replies: 11
- Views: 1072
Re: When to use + sign
It's good notation for whenever you're dealing with change in a value.
- Sat Feb 10, 2018 2:54 pm
- Forum: Balancing Redox Reactions
- Topic: Problem 14.1
- Replies: 2
- Views: 295
Re: Problem 14.1
C is oxidized from 2- to 1- the same way. C 2 H 5 OH has 0 charge. H has a charge of 1+, O has a charge of 2-. This gives H 5 OH a charge of 4. Each C in C 2 needs to have a charge of 2- to cancel out the 4+ charge. When you do this process for the product, C has a charge of 1- so it is oxidized fro...
- Sat Feb 10, 2018 2:49 pm
- Forum: Balancing Redox Reactions
- Topic: Problem 14.1
- Replies: 2
- Views: 295
Re: Problem 14.1
Cr2O72- has a charge of 2-. O usually has a charge of 2- and -2 x 7 = -14. So the charge of two Cr when added to -14 needs to equal -2. This makes the charge of Cr be 6+ because 2(6) -14 = -2.
- Fri Feb 02, 2018 7:08 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: 9.7 vs 9.13
- Replies: 4
- Views: 405
Re: 9.7 vs 9.13
When you use the equation with specific heat capacity and constant volume or pressure you use (5/2)R. For monatomic ideal gases Cv=(3/2)R and Cp=(5/2)R. For diatomic gases, such as nitrogen in this problem, Cv=(5/2)R and Cp=(7/2)R
- Wed Jan 31, 2018 4:20 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: 9.7 vs 9.13
- Replies: 4
- Views: 405
Re: 9.7 vs 9.13
You actually use C in the second equation too when dealing with temperature changes, but the different entropy equations come from ∆S=q/T so they basically function the same. C depends on whether you are calculating constant pressure or constant volume and is actually a multiple of R. In 9.13, you h...
- Wed Jan 31, 2018 4:06 pm
- Forum: Calculating Work of Expansion
- Topic: Irreversible Work Chart
- Replies: 5
- Views: 486
Re: Irreversible Work Chart
If you look on page 275 of the textbook, there is an example problem that shows that the pressure did drop by cooling. However, this takes place before any of the expansion work is actually done, so it is a vertical line. This line could have just as easily not been there, but it is just trying to s...
- Sat Jan 27, 2018 10:09 am
- Forum: Calculating Work of Expansion
- Topic: Irreversible Work Chart
- Replies: 5
- Views: 486
Re: Irreversible Work Chart
The vertical brown line is the drop in the pressure. This was probably achieved by cooling at a constant volume (delta V = 0) so there is no work done.
- Tue Jan 23, 2018 6:34 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Concepts and Equations
- Replies: 2
- Views: 166
Re: Concepts and Equations
It should be a positive sign in the equation you gave, but you can get this equation from ΔU = q + w which is on the constants and equations sheet. Under constant pressure, ΔU = qp + w. qp = ΔH and w = -PΔV. If you plug those in and rearrange, you get the equation ΔH = ΔU + PΔV.
- Mon Jan 22, 2018 11:29 pm
- Forum: Phase Changes & Related Calculations
- Topic: Self Test 8.1
- Replies: 1
- Views: 220
Re: Self Test 8.1
What have you been getting for your answer? I got w = -0.94 kJ.
- Sat Jan 20, 2018 1:15 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: change in internal energy
- Replies: 4
- Views: 325
Re: change in internal energy
Change in internal energy can be equaled to heat transfer when there is no work done (volume of reactants = volume of products). Change of internal energy is equal to work done when there is no heat exchanged. This can be seen in the equation ΔU = q + w.
- Sat Jan 20, 2018 1:11 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: ΔH = ΔU + PΔV
- Replies: 2
- Views: 1619
Re: ΔH = ΔU + PΔV
The equation ΔU = q + w comes from the fact that the internal energy of a closed system can be changed by heating/cooling or compression/expansion. Under constant pressure, ΔU = qp + w. qp = ΔH and w = -PΔV. If you plug those in and rearrange, you get the equation ΔH = ΔU + PΔV.
- Sat Jan 20, 2018 1:04 pm
- Forum: Phase Changes & Related Calculations
- Topic: Why does steam cause severe burns?
- Replies: 4
- Views: 471
Re: Why does steam cause severe burns?
Water and steam can both exist at 100 degrees F. However steam also contains more heat/energy than water because of the enthalpy of vaporization needed to turn water into steam. When steam comes into contact with the skin, it is exothermic and releases heat. This also releases the heat that was nece...
- Thu Jan 11, 2018 6:32 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.57
- Replies: 1
- Views: 229
Re: 8.57
The c makes it stand for enthalpy of combustion. You have to write a reaction for the combustion of each of the compounds (by adding O2) so that you can find out their enthalpy of formation. From there, you can use Hess's Law to calculate enthalpy for the hydrogenation of ethyne to ethane reaction.
- Wed Jan 10, 2018 11:49 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Closed System
- Replies: 7
- Views: 779
Re: Closed System
Had to look up how a refrigerator works, but basically there is a compressor, and coil on the outside and inside of the fridge. Hot gaseous coolant is compressed and pushed into the coil on the outside of the fridge. When it meets the cooler temperature of a kitchen, it becomes a liquid. In liquid f...
- Wed Jan 10, 2018 11:30 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bond Enthalpies
- Replies: 6
- Views: 2356
Re: Bond Enthalpies
Also, the products side is negative because energy is released when new bonds are formed.

