Search found 50 matches
- Wed Mar 14, 2018 3:12 pm
- Forum: Phase Changes & Related Calculations
- Topic: 15.85
- Replies: 2
- Views: 457
Re: 15.85
Unimolecular means that the reaction involves one molecule, bimolecular means the reaction involves two molecules, and termolecular means the reaction involves three molecules.
- Wed Mar 14, 2018 3:09 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Catalyst and Intermediate
- Replies: 5
- Views: 788
Re: Catalyst and Intermediate
A catalyst does not change the reactants and products of a reaction - it just changes the pathway. So you should be able to have catalysts and intermediates in the same reaction.
- Wed Mar 14, 2018 3:06 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: k' reverse reaction constant
- Replies: 6
- Views: 1100
Re: k' reverse reaction constant
It's just the notation - it distinguishes k from k'. I don't think there's anything special about it.
- Fri Mar 09, 2018 8:31 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3636807
Re: Post All Chemistry Jokes Here
Q: What kind of fish is made out of 2 sodium atoms?
A: 2 Na
A: 2 Na
- Fri Mar 09, 2018 8:31 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3636807
Re: Post All Chemistry Jokes Here
Q: What do you call a wheel made of iron?
A: A ferrous wheel.
A: A ferrous wheel.
- Fri Mar 09, 2018 8:30 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3636807
Re: Post All Chemistry Jokes Here
Q: What do you call a clown who's in jail?
A: A silicon.
A: A silicon.
- Wed Feb 28, 2018 4:41 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: When do we calculate for k
- Replies: 3
- Views: 490
Re: When do we calculate for k
k is based on experimental data, so if you're given data you are probably expected to calculate it. If not, I think you can use the table.
- Wed Feb 28, 2018 2:26 pm
- Forum: Zero Order Reactions
- Topic: Using Half Life with Zero Order
- Replies: 2
- Views: 429
Re: Using Half Life with Zero Order
On page 631, the textbook also says that the half-life is not used for second order reactions. Maybe this is because the half-life depends on the initial concentration for zero and second order reactions? This would make it difficult to compare reactions.
- Wed Feb 28, 2018 2:13 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: 15.19
- Replies: 2
- Views: 495
Re: 15.19
From experiment 2:
17.4 mmol/L*s = k(2.5 mmol/L)(1.25^2 mmol^2/L^2)(1.25^2 mmol^2/L^2)
k = 17.4 mmol/L*s /(1.25^2*1.25^2*2.5) mmol^5/L^5
The mmol and L cancel, so you have
k = 17.4/s /(1.25^2*1.25^2*2.5) L^4/mmol^4
1 mmol = 10^3 mol, so
k = 17.4/(1.25^2*1.25^2*2.5)* 10^12 L^4/mol^4*s
17.4 mmol/L*s = k(2.5 mmol/L)(1.25^2 mmol^2/L^2)(1.25^2 mmol^2/L^2)
k = 17.4 mmol/L*s /(1.25^2*1.25^2*2.5) mmol^5/L^5
The mmol and L cancel, so you have
k = 17.4/s /(1.25^2*1.25^2*2.5) L^4/mmol^4
1 mmol = 10^3 mol, so
k = 17.4/(1.25^2*1.25^2*2.5)* 10^12 L^4/mol^4*s
- Thu Feb 22, 2018 7:45 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Nernst Equation to find pH
- Replies: 6
- Views: 10197
Re: Nernst Equation to find pH
At standard conditions, you can use the formula logQ) to find the pH. The formula for ph is pH = -log[H+}, so you can find the concentration of hydrogen ions by the Nearst equation.The formula above is already converted from ln to log.
to find the pH. The formula for ph is pH = -log[H+}, so you can find the concentration of hydrogen ions by the Nearst equation.The formula above is already converted from ln to log.
- Thu Feb 22, 2018 7:41 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: swapping signs of E values
- Replies: 8
- Views: 3694
Re: swapping signs of E values
If you use the formula 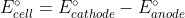 you do not need to change the sign of
you do not need to change the sign of 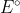 for either half reaction because the minus sign does it for you.
for either half reaction because the minus sign does it for you.
- Thu Feb 22, 2018 7:38 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Homework 14.9
- Replies: 1
- Views: 302
Re: Homework 14.9
In order to use the formula 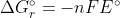 , you can solve for n by writing out the oxidation and reduction half reactions and balancing them.
, you can solve for n by writing out the oxidation and reduction half reactions and balancing them.
- Wed Feb 14, 2018 1:13 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Temperature Relation
- Replies: 2
- Views: 389
Re: Temperature Relation
Relationship between T and delta U: \Delta U = q (assuming no work is done) If you increase heat added (increase q) then delta U increases. Relationship between T and delta S: delta S \Delta S __{T_{1} \rightarrow T_{2}} = nC(\ln T_{2} - \ln T_{1}) If you look at the natural log graph: If T_...
- Wed Feb 14, 2018 12:30 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Difference between delta S and delta S naught
- Replies: 2
- Views: 8248
Re: Difference between delta S and delta S naught
The third law of thermodynamics says that the total entropy is always increasing, so delta S total must be positive. The standard enthalpy for a reaction can be positive or negative depending on the reaction. For example, building polymers from monomers has a negative delta S (the complexity is incr...
- Wed Feb 14, 2018 12:29 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: 9.73 and delta S tot
- Replies: 1
- Views: 346
Re: 9.73 and delta S tot
The third law of thermodynamics says that the total entropy is always increasing, so delta S total must be positive. The standard enthalpy for a reaction can be positive or negative depending on the reaction. For example, building polymers from monomers has a negative delta S (the complexity is incr...
- Wed Feb 07, 2018 7:02 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: K to Kc equation
- Replies: 2
- Views: 426
Re: K to Kc equation
Kc is the equilibrium constant in terms of molar concentrations. See section 11.6 in the textbook.
- Wed Feb 07, 2018 6:59 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: 9.25
- Replies: 2
- Views: 437
Re: 9.25
The question says "what might its residual molar entropy be?" so you would use avogadro's constant.
- Wed Feb 07, 2018 6:57 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 9.19
- Replies: 1
- Views: 333
Re: 9.19
Because entropy is a state function and you know the vaporization at 100 degrees C, to find the standard entropy of vaporization at 85 degrees C you can heat the water to 100 degrees C, vaporize it, then cool it to 85 degrees C. This will give you the standard entropy of vaporization at 85 degrees C.
- Thu Feb 01, 2018 6:33 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Negative delta s value
- Replies: 4
- Views: 11662
Re: Negative delta s value
A negative delta S corresponds to a spontaneous process when the magnitude of T * delta S is less than delta H (which must be negative). This is because delta G must be negative, and delta G = delta H - (T * delta S). A negative delta S would mean that the products have a lower entropy than the reac...
- Thu Feb 01, 2018 6:29 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Spontaneous
- Replies: 14
- Views: 1994
Re: Spontaneous
Reactions are spontaneous when delta G is negative. delta G = delta H - (T * delta S) Releasing heat is favored, and increasing entropy is favored (for example, liquid to gas - especially favored at higher temperatures). You have to consider both cases to make a conclusion about the spontaneity of a...
- Wed Jan 31, 2018 4:22 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: State functions [ENDORSED]
- Replies: 7
- Views: 778
Re: State functions [ENDORSED]
Delta S is also a state function.
- Tue Jan 23, 2018 8:09 pm
- Forum: Phase Changes & Related Calculations
- Topic: Phase Changes and Energy
- Replies: 2
- Views: 345
Re: Phase Changes and Energy
I think s->l->g requires energy and g->l->s releases energy.
- Tue Jan 23, 2018 8:05 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Problem 8.51
- Replies: 2
- Views: 564
Re: Problem 8.51
You use the enthalpies of formation to calculate delta H, then you have to convert kJ/mol TNT to kJ/L, which is why you need to know the density of TNT.
- Tue Jan 23, 2018 8:03 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 8.51
- Replies: 1
- Views: 288
Re: 8.51
The first step is to balance the equation that they describe in the first sentence. CO + H2O -> CO2 + H2 Then you know that the heat absorbed by the calorimeter is equal to the heat released by the reaction. qcal = -qrxn And delta U = q because the reaction occurred in a bomb calorimeter (w = 0 beca...
- Thu Jan 18, 2018 6:05 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Why is using bond enthalpies the least accurate method of finding reaction enthalpies?
- Replies: 5
- Views: 10152
Re: Why is using bond enthalpies the least accurate method of finding reaction enthalpies?
Bond enthalpies are averages of many bonds.
- Thu Jan 18, 2018 3:38 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.51
- Replies: 1
- Views: 211
Re: 8.51
I believe that the sign is positive because the definition of enthalpy density is the enthalpy released per liter, so adding a negative sign would mean energy is absorbed.
- Mon Jan 15, 2018 7:31 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Q. 8.39
- Replies: 2
- Views: 313
Re: Q. 8.39
I would draw the phase change chart for water first. The first phase change is at 0 degrees and the second is at 100 degrees. If you start at 0 degrees (ice) and go to 20 degrees (liquid), you need to consider both the heat required to change ice to a liquid and the heat required to raise the temper...
- Fri Jan 12, 2018 10:26 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.61
- Replies: 4
- Views: 339
Re: 8.61
Like balancing chemical reactions, I recommend manipulating the equations that have the least number of the same molecule first, and the should build on it.
- Fri Jan 12, 2018 10:22 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.49
- Replies: 5
- Views: 395
Re: 8.49
What is the difference between internal energy and enthalpy? H = U + PV. Enthalpy is equal to internal energy plus the product of pressure and volume, so internal energy is included in the enthalpy calculation. Also, internal energy has to do with the kinetic and potential energies of molecules in ...
- Fri Jan 12, 2018 10:09 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: 8.1 part c [ENDORSED]
- Replies: 8
- Views: 1164
- Thu Dec 07, 2017 6:53 pm
- Forum: Amphoteric Compounds
- Topic: Amphiprotic [ENDORSED]
- Replies: 5
- Views: 1003
Re: Amphiprotic [ENDORSED]
Amphiprotic is the ability of a molecule to both accept and donate protons.
Amphoteric is the ability of a molecule to react with both acids and bases.
See page 469 in the textbook.
Amphoteric is the ability of a molecule to react with both acids and bases.
See page 469 in the textbook.
- Thu Dec 07, 2017 6:43 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: 12.25
- Replies: 1
- Views: 289
Re: 12.25
I believe the acid/base will not dissociate unless it is in some sort of solvent. In this case, the problem states Ba(OH)2 is in an aqueous solution. Ba(OH)2 is a strong acid, so you do not need to set up an ice box, and you are not given Kb. Because Ba(OH)2 is a strong base, we assume it is 100% io...
- Wed Dec 06, 2017 11:43 am
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: 12.49
- Replies: 5
- Views: 1343
Re: 12.49
Is there any way to figure out this problem by looking at the Lewis structures?
- Tue Nov 28, 2017 6:51 pm
- Forum: Naming
- Topic: (EDTA) and (en) Q17.37
- Replies: 1
- Views: 354
Re: (EDTA) and (en) Q17.37
If you look at table 4, the full name of the ligand is given and the abbreviation is in parentheses: ethylenediamine (en) enthylenediaminetetraacetato (edta) After the parentheses is a symbol that corresponds to one of the footnotes telling you whether the ligand is bidentrate, tridentate, or hexade...
Re: 17.33 (a)
The carbons and nitrogens form a long chain, with the hydrogens bonded to each atom. The order in which the atoms are written gives the order of the chain, and because there are two identical segments, the molecule can be drawn as symmetrical. If you count the number of available valence electrons, ...
- Tue Nov 28, 2017 6:13 pm
- Forum: Naming
- Topic: naming with anions
- Replies: 2
- Views: 452
Re: naming with anions
This makes sense because in order for it to be a neutral compound, there must be three chlorine atoms. The chromium atom has a +3 charge, so there must be three chlorine atoms, each with a -1 charge. "Trichloride would be redundant."
- Mon Nov 13, 2017 7:47 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: AXE
- Replies: 3
- Views: 447
Re: AXE
The subscript of E is the number of lone pairs around the central atom. You would have to draw the Lewis structure.
- Mon Nov 13, 2017 4:45 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: lone pairs in trigonal bipyramidal shape
- Replies: 2
- Views: 1783
Re: lone pairs in trigonal bipyramidal shape
See figure 4.5 in the textbook (page 115): "If the lone pairs had taken the axial positions, they would have been at 90 degree angles from the equatorial positions, resulting in greater repulsion."
- Mon Nov 13, 2017 4:39 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Question 4.25
- Replies: 2
- Views: 569
Re: Question 4.25
You also have to think about the shape of the molecule. For example, in part (a) (CH2Cl2), the molecule has a tetrahedral shape, so the dipole moments do not cancel, even though they look they could in the Lewis structure -> The molecule is polar.
- Mon Nov 13, 2017 4:10 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Book Questions 4.1&4.2
- Replies: 4
- Views: 476
Re: Book Questions 4.1&4.2
Page 118 of the textbook provides good graphics for this concept. For the linear model, the molecule can either have the formula AX2 or AX2E3, so it could have lone pairs around the central atom.
- Mon Nov 13, 2017 3:41 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Question 4.13 a
- Replies: 2
- Views: 382
Re: Question 4.13 a
If you look on page 118 in the textbook, there are really good diagrams that show the shapes. If you think about a molecule with 5 regions of electron density and no lone pairs, the shape is trigonal bipyramidal. Then if you replace the 3 atoms in the plane with 3 lone pairs, the shape is linear.
- Mon Nov 13, 2017 11:24 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 4.1
- Replies: 5
- Views: 597
Re: 4.1
It is possible for (b) to have lone pairs if it has 3 lone pairs around the central atom. It will still be a linear shape. See page 118 of the book for a graphic.
- Mon Nov 13, 2017 10:20 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Organic vs inorganic molecules
- Replies: 3
- Views: 462
Re: Organic vs inorganic molecules
I also believe that organic molecules are usually chains or rings.
- Mon Nov 13, 2017 10:11 am
- Forum: Lewis Structures
- Topic: Bond Lengths [ENDORSED]
- Replies: 3
- Views: 516
Re: Bond Lengths [ENDORSED]
This could be because of resonance. The molecule is an average of the resonance structures, so the bond length is an average as well.
- Tue Oct 24, 2017 2:45 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Question 51
- Replies: 1
- Views: 240
Re: Question 51
I looked at the last block the electrons occupy. For example, Bi has 3 electrons in the 6p-block, and because of Hund's Rule and the Pauli Exclusion Principle, each electron will occupy a separate subshell (px, py, pz), so there are three unpaired electrons.
- Tue Oct 24, 2017 2:39 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Ground State naming of elements
- Replies: 1
- Views: 197
Re: Ground State naming of elements
I believe we are expected to know the s-block, p-block, and first row of the transition metals.
- Wed Oct 18, 2017 11:06 pm
- Forum: Properties of Light
- Topic: Angstrom
- Replies: 3
- Views: 582
Re: Angstrom
1 Ångström is 10^-10 meters. Bond lengths are about 1 Ångström.
- Sun Oct 15, 2017 6:15 pm
- Forum: Photoelectric Effect
- Topic: Wavelike properties [ENDORSED]
- Replies: 4
- Views: 508
Re: Wavelike properties [ENDORSED]
If it creates a diffraction pattern (like the electrons did in the slit experiment), it must have wavelike properties.
Or, based on De Broglie's relation, any moving particle with momentum has wavelike properties, but sometimes the wavelength is so small there are no measurable wavelike properties.
Or, based on De Broglie's relation, any moving particle with momentum has wavelike properties, but sometimes the wavelength is so small there are no measurable wavelike properties.
- Tue Oct 10, 2017 2:58 pm
- Forum: SI Units, Unit Conversions
- Topic: Significant Figures [ENDORSED]
- Replies: 2
- Views: 385
Re: Significant Figures [ENDORSED]
I believe this rule is to eliminate rounding errors in a multi-step problem (50% is rounded up and 50% is rounded down). However, in order to be as precise as possible you should not round your answer until the very end of the problem.
- Tue Oct 10, 2017 2:49 pm
- Forum: Properties of Light
- Topic: Converting to Different Units
- Replies: 3
- Views: 456
Re: Converting to Different Units
In case you do not have them on hand, the prefixes are: centi 10^-2 mili 10^-3 micro 10^-6 nano 10^-9 An easy way to remember the prefixes is that they decrease by three powers every "step," with the exclusion of centi (which you can remember by centipede or centimeter, although I do not b...

