Search found 73 matches
- Thu Mar 15, 2018 10:51 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Negative Ea
- Replies: 3
- Views: 598
- Thu Mar 15, 2018 12:06 am
- Forum: Administrative Questions and Class Announcements
- Topic: Lecture Friday
- Replies: 4
- Views: 711
Re: Lecture Friday
At the end of class
- Thu Mar 15, 2018 12:05 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: 15.85
- Replies: 1
- Views: 251
Re: 15.85
An activated complex is like a transition state, so you would draw out a transition state for the molecules.
- Thu Mar 15, 2018 12:03 am
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: reversible and perfect system
- Replies: 1
- Views: 435
Re: reversible and perfect system
For reversible reactions, deltaS total is 0. For perfect crystals, entropy is 0 at 0K.
- Tue Mar 13, 2018 8:29 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Lecture Friday
- Replies: 4
- Views: 711
Re: Lecture Friday
Nope
- Tue Mar 13, 2018 7:44 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Pre-Equilibrium Method Conditions
- Replies: 2
- Views: 386
Re: Pre-Equilibrium Method Conditions
It has to be a multi-step reaction where a fast step comes before a slow step.
- Tue Mar 13, 2018 7:44 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: steady-state
- Replies: 1
- Views: 279
Re: steady-state
We only need to do pre-equilibrium approach. We won't be doing the steady state approximation.
- Mon Mar 12, 2018 10:09 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.49
- Replies: 1
- Views: 266
8.49
For this question, why can we assume that the temperature is 298 K?
- Mon Mar 12, 2018 2:10 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Adsorption
- Replies: 3
- Views: 462
Re: Adsorption
Does adsorption only happen for a heterogeneous catalyst?
- Mon Mar 12, 2018 2:09 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Activation Energy Temperature Dependence
- Replies: 5
- Views: 1194
Re: Activation Energy Temperature Dependence
Does what you described have to do with the frequency factor at all?
- Mon Mar 12, 2018 2:04 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Problem 15.61
- Replies: 5
- Views: 635
Re: Problem 15.61
Do you know where A went though?
- Mon Mar 12, 2018 1:59 pm
- Forum: *Alcohols
- Topic: Functional Groups on Final? [ENDORSED]
- Replies: 6
- Views: 2096
Re: Functional Groups on Final? [ENDORSED]
He said whatever is discussed in lecture will be on final.
- Mon Mar 12, 2018 1:58 pm
- Forum: *Alcohols
- Topic: Functional group in final [ENDORSED]
- Replies: 1
- Views: 994
Re: Functional group in final [ENDORSED]
Only the functional groups in class. He said these are the most common.
- Mon Mar 12, 2018 1:32 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Catalysts
- Replies: 3
- Views: 444
Re: Catalysts
I believe in can be in the rate law.
- Mon Mar 12, 2018 1:31 am
- Forum: Administrative Questions and Class Announcements
- Topic: Organic Chem on Final
- Replies: 9
- Views: 1439
Re: Organic Chem on Final
Since we went over Sn2 reactions in class, I think some ochem will be on the final.
- Mon Mar 12, 2018 1:29 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Ways to approach reaction mechanism
- Replies: 2
- Views: 375
Re: Ways to approach reaction mechanism
The direct computation method has a lot of advanced math that computers do, so we will not use it. We only need to know how to do the pre-equilibrium method which is where a slow step causes a bottleneck of the products from the step before, creating a pre-equilibrium.
- Mon Mar 12, 2018 1:24 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Example 15.7
- Replies: 2
- Views: 304
Re: Example 15.7
We do not need to know how to do the steady state approximation, but we do need to use pre-equilibrium conditions (both should give the same answer).
- Mon Mar 12, 2018 1:23 am
- Forum: General Rate Laws
- Topic: Rate law constant
- Replies: 4
- Views: 543
Re: Rate law constant
Since rates are always positive values, the rate constant k is always positive.
- Sun Mar 04, 2018 8:20 pm
- Forum: First Order Reactions
- Topic: radioactive decay
- Replies: 4
- Views: 570
Re: radioactive decay
Does this mean that radioactive decay only works for a reaction if there's one reactant since it's a first order reaction?
- Sun Mar 04, 2018 8:00 pm
- Forum: General Rate Laws
- Topic: Naming Clarification
- Replies: 3
- Views: 454
Re: Naming Clarification
Yes! They're the same.
- Thu Mar 01, 2018 9:50 am
- Forum: General Rate Laws
- Topic: Using more than one species [ENDORSED]
- Replies: 1
- Views: 287
Re: Using more than one species [ENDORSED]
Writing rate laws means using concentration of the reactants and a rate constant k. You will have to determine the order of each reactant to get the exponent and if it is 0 order, that reactant won't show up.
- Wed Feb 21, 2018 9:26 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Metal on ends of galvanic cell
- Replies: 2
- Views: 361
Re: Metal on ends of galvanic cell
Usually platinum is used as an inert metal electrode.
- Wed Feb 21, 2018 9:25 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Notation
- Replies: 2
- Views: 361
Re: Notation
Comma is for same phase and solid line is for different phase.
- Wed Feb 21, 2018 9:24 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cathode vs Anode
- Replies: 4
- Views: 637
Re: Cathode vs Anode
As long as solids are on the outsides, it does not matter.
- Wed Feb 21, 2018 9:23 pm
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: Electrode v. electrolyte
- Replies: 1
- Views: 419
Re: Electrode v. electrolyte
The electrode is the solid metal that conducts electricity, and the electrolyte is the aqueous solution with ions.
- Wed Feb 21, 2018 9:22 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: When writing cell diagrams, how do you know when to include an additional element at the electrode?
- Replies: 5
- Views: 593
Re: When writing cell diagrams, how do you know when to include an additional element at the electrode?
When the redox reaction has no solid that can act as the electrode, you will need a platinum electrode.
- Mon Feb 19, 2018 3:59 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 14.15A
- Replies: 4
- Views: 528
Re: 14.15A
For this problem the cell diagram in the solutions manual divides Ag(s) and AgBr(s) on the anode side with a vertical line. Can someone explain why they aren't separated by a comma even though they're the same phase?
- Fri Feb 16, 2018 9:22 pm
- Forum: Balancing Redox Reactions
- Topic: Salt Bridge Versus Pourous Disk
- Replies: 6
- Views: 816
Re: Salt Bridge Versus Pourous Disk
Both allow exchange of counter ions to maintain the charge difference while separating the atoms or elements that are actually involved in the redox reactions.
- Fri Feb 16, 2018 9:10 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Explaining 14.4 (Cell potential and gibbs)
- Replies: 2
- Views: 308
Re: Explaining 14.4 (Cell potential and gibbs)
I think that Ecell when we calculate it represents the maximum potential difference. SInce this potential difference can be used like a battery, we are talking about the maximum possible difference, but real-world batteries would not actually produce this much voltage.
- Fri Feb 16, 2018 9:08 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Potential in Galvanic/Voltaic Cells
- Replies: 2
- Views: 233
Re: Potential in Galvanic/Voltaic Cells
I think that since cell potential is also sensitive to the concentrations of reactants and products, you can set up a cell that will have a voltage difference because of differences in concentration. So even though both sides have the same molecule or element, the difference in concentrations will c...
- Wed Feb 07, 2018 9:27 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bonds Broken - Bonds Formed?
- Replies: 2
- Views: 2233
Re: Bonds Broken - Bonds Formed?
It takes energy to break bonds (positive enthalpy) and releases energy to form bonds (negative enthalpy) and then you add those enthalpies together. You could also do broken-formed but make sure you use bond enthalpies directly.
- Wed Feb 07, 2018 9:25 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 9.47
- Replies: 1
- Views: 266
Re: 9.47
For a reversible process, the reaction is assumed to be at equilibrium which means deltaS of the universe is zero because there is no entropy change at equilibrium.
- Wed Feb 07, 2018 9:16 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Spontaneity
- Replies: 9
- Views: 1287
Re: Spontaneity
Negative delta G is spontaneous because free energy goes from a higher energy state to a lower energy state which is favorable.
- Sun Feb 04, 2018 8:24 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 9.13
- Replies: 7
- Views: 1013
Re: 9.13
Lavelle's formula sheet gives the approximation for monatomic ideal gases only, which is why it says 3/2R. For diatomic ideal gases, the approximation is 5/2R.
- Tue Jan 30, 2018 5:10 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 9.13
- Replies: 7
- Views: 1013
Re: 9.13
I think for the first part of the problem we have to use nR but the second part is the one where we have to use C. And on the solution errors page it says to use Cv but it also says 5/2R
- Tue Jan 30, 2018 5:00 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 9.13
- Replies: 7
- Views: 1013
Re: 9.13
How do we know whether to use Cv or Cp though? I thought the volume and pressure were both changing???
- Sun Jan 28, 2018 4:11 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Pressure in Reversible Pathways [ENDORSED]
- Replies: 1
- Views: 237
Pressure in Reversible Pathways [ENDORSED]
Can someone please explain why pressure decreases in a reversible pathway? I'm confused by the graph that was in the notes comparing reversible and irreversible pathways. Thanks!
- Fri Jan 26, 2018 12:26 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Formula confusion [ENDORSED]
- Replies: 2
- Views: 191
Formula confusion [ENDORSED]
Can someone explain the difference between 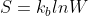 and
and 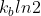 ?
?
- Fri Jan 26, 2018 12:02 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: State properties of heat and enthalpy
- Replies: 2
- Views: 322
State properties of heat and enthalpy
Why is enthalpy a state property and heat not a state property?
- Fri Jan 19, 2018 4:25 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: P delta V is significant?
- Replies: 4
- Views: 1033
P delta V is significant?
Can someone explain why P delta V is insignificant when there's a reaction with constant P that involves solids and liquids? Thanks!
- Thu Jan 18, 2018 4:04 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Adiabatic [ENDORSED]
- Replies: 8
- Views: 1043
Adiabatic [ENDORSED]
My TA mentioned this during discussion, but can someone explain what adiabatic means?
- Thu Jan 18, 2018 3:57 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Reversible, Irreversible
- Replies: 4
- Views: 469
Reversible, Irreversible
Can someone explain the difference between reversible and irreversible reactions?
- Thu Jan 11, 2018 4:50 pm
- Forum: Phase Changes & Related Calculations
- Topic: Temperature during phase changes [ENDORSED]
- Replies: 4
- Views: 567
Temperature during phase changes [ENDORSED]
Why does the temperature stay the same during phase changes?
- Thu Jan 11, 2018 4:31 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bond Enthalpies vs. Standard Enthalpies of Formation
- Replies: 2
- Views: 215
Bond Enthalpies vs. Standard Enthalpies of Formation
What's the difference between bond enthalpies vs standard enthalpies of formation?
- Thu Jan 11, 2018 4:25 pm
- Forum: Phase Changes & Related Calculations
- Topic: ∆Hsub= ∆Hfus+ ∆Hvap
- Replies: 4
- Views: 3360
∆Hsub= ∆Hfus+ ∆Hvap
Can someone explain me how ∆Hsub= ∆Hfus+ ∆Hvap?
- Sat Dec 09, 2017 12:42 am
- Forum: Lewis Acids & Bases
- Topic: CaO (s) in H2O
- Replies: 1
- Views: 2024
Re: CaO (s) in H2O
CaO is a strong base, so it dissociates into Ca2+ and O2-. The O2- accepts a proton from water to form OH-. Since this creates a basic solution, CaO is a base.
- Sat Dec 09, 2017 12:39 am
- Forum: Amphoteric Compounds
- Topic: Amphoteric [ENDORSED]
- Replies: 4
- Views: 960
Re: Amphoteric [ENDORSED]
Metal oxides tend to be bases, while non-metal oxides tend to be acids. There is a diagonal band similar to the metalloid band and those oxides are amphoteric. I think you can find that in the textbook.
- Sat Dec 09, 2017 12:38 am
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: pH Significant Figures
- Replies: 1
- Views: 196
Re: pH Significant Figures
I think only digits after the decimal place are considered significant. For example, pH of 4.23 would be 2 sig figs.
- Tue Dec 05, 2017 12:21 am
- Forum: Calculating the pH of Salt Solutions
- Topic: Salt + Water?
- Replies: 2
- Views: 585
Re: Salt + Water?
In lecture, Dr. Lavelle said "almost always" so there are probably some exceptions. For example, autoprotolysis where water acts as an acid and a base does not produce a salt. But for the most part, acids and bases will react to form salt and water.
- Tue Dec 05, 2017 12:08 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Endothermic v. Exothermic Reactions
- Replies: 9
- Views: 2661
Re: Endothermic v. Exothermic Reactions
In endothermic reactions, heat is consumed or absorbed to move the reaction forward. In exothermic reactions, heat is released or given off. You can think of endothermic reactions as having heat as a reactant, whereas exothermic reactions have heat as a product.
- Sat Dec 02, 2017 10:40 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Molecular Orbital Theory
- Replies: 4
- Views: 443
Re: Molecular Orbital Theory
It is not on the syllabus or any of the outlines or homeworks, so we do not need to know it for the final:)
- Sat Dec 02, 2017 10:35 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: difference in acids
- Replies: 3
- Views: 558
Re: difference in acids
Bronsted vs Lewis definitions are just focusing on different aspects of the molecule to determine acidity and basicity. Using either definition will lead to the same answer. For example, a Lewis acid is the same as a Bronsted acid, but if you say Lewis acid you are focusing on the ability of the mol...
- Sat Dec 02, 2017 10:33 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Polydentates
- Replies: 1
- Views: 413
Re: Polydentates
It might help to draw it out, but in general, I usually look for nitrogens with lone pairs and oxygens with a negative formal charge because these most commonly act as binding sites in coordination compounds. Then I count how many there are, and that is how I determine what kind of dentate it is. Ho...
- Fri Nov 24, 2017 12:13 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Partial Pressure or Concentration
- Replies: 3
- Views: 424
Partial Pressure or Concentration
How do I know when to use partial pressure or concentration when calculating the equilibrium constant?
- Sun Nov 19, 2017 5:09 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Ligands
- Replies: 2
- Views: 269
Ligands
Why are ligands Lewis bases?
- Sun Nov 19, 2017 5:06 pm
- Forum: Hybridization
- Topic: Hybrid orbitals and bonds
- Replies: 3
- Views: 282
Hybrid orbitals and bonds
How do hybridized orbitals relate to sigma and pi bonds?
- Sun Nov 12, 2017 3:23 pm
- Forum: Dipole Moments
- Topic: Dipole Moments
- Replies: 2
- Views: 397
Dipole Moments
How do I know when dipole moments cancel?
- Sun Nov 12, 2017 3:08 pm
- Forum: Lewis Structures
- Topic: Midterm Question CH3SH
- Replies: 5
- Views: 1063
Re: Midterm Question CH3SH
Yes I got that too.
- Sun Nov 05, 2017 8:37 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: HW 3.83
- Replies: 7
- Views: 3156
Re: HW 3.83
Polarizing power and polarizability are opposites. Polarizing power refers to the cation's ability to pull electrons toward it, so when the atom is smaller, there are less electrons, so the anion will be closer to the nucleus and feel a stronger pull. Polarizability is how easily an atom's electron ...
- Sun Nov 05, 2017 8:30 pm
- Forum: Lewis Structures
- Topic: Beryllium and Lithium
- Replies: 2
- Views: 418
Beryllium and Lithium
Why don't Lithium and Beryllium have full octet when drawing Lewis structures?
- Fri Oct 27, 2017 10:25 pm
- Forum: Significant Figures
- Topic: How to solve #18 Post-Assessment [ENDORSED]
- Replies: 1
- Views: 381
Re: How to solve #18 Post-Assessment [ENDORSED]
You would have to calculate what exactly 1% of the hydrogen radius is, and then use that as your deltaX (uncertainty in position) in the Heisenberg uncertainty formula. Then you would solve for deltaV which is the uncertainty in velocity. Hope this helps! :)
- Fri Oct 27, 2017 10:21 pm
- Forum: Lewis Structures
- Topic: Lewis Structures- resonance [ENDORSED]
- Replies: 4
- Views: 437
Re: Lewis Structures- resonance [ENDORSED]
For the nitrate example, any of the three resonance structures are correct if the questions just asks for a lewis structure. However, we just have to understand that the real structure is a blend of the three. For other examples though, we would have to look at formal charges to determine which is t...
- Fri Oct 27, 2017 10:18 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Electron affinity
- Replies: 4
- Views: 432
Re: Electron affinity
Nuclear charge plays a role because higher nuclear charge will have a greater pull on electrons which means higher electron affinity, which is why electron affinity increases going across a row. The almost-full valence shell is another way of thinking about it, because atoms want to have noble gas c...
- Sat Oct 21, 2017 10:59 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: orbitals an shells [ENDORSED]
- Replies: 5
- Views: 1129
Re: orbitals an shells [ENDORSED]
Can you clarify the difference between shells and subshells?
- Sat Oct 21, 2017 10:46 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: s-electrons and p-electrons [ENDORSED]
- Replies: 2
- Views: 433
s-electrons and p-electrons [ENDORSED]
Can someone explain why s-electrons have a lower energy than p-electrons? Confused because their energy differs even though they're in the same shell??
- Sun Oct 15, 2017 12:14 pm
- Forum: *Black Body Radiation
- Topic: Black body for quiz 2 [ENDORSED]
- Replies: 11
- Views: 1764
Re: Black body for quiz 2 [ENDORSED]
I am pretty sure Dr. Lavelle said we would not be covering black bodies, so you will not need to know Wien's law or Stephen Boltzmann law.
- Sun Oct 15, 2017 12:12 pm
- Forum: Properties of Light
- Topic: Concept // Wave-like Behavior [ENDORSED]
- Replies: 4
- Views: 510
Re: Concept // Wave-like Behavior [ENDORSED]
I believe the way light reflects, refracts, diffracts, and interferes all point to its wave-like characteristics.
- Sun Oct 15, 2017 12:10 pm
- Forum: DeBroglie Equation
- Topic: Units for Mass [ENDORSED]
- Replies: 3
- Views: 464
Re: Units for Mass [ENDORSED]
When doing the work for some of the problems, it will be necessary to change units to kilograms depending on what units the constants have that you use in your equations. But for finals answers, you can leave your answer in grams or kilograms as long as you have the right sig figs and right conversi...
- Sun Oct 15, 2017 12:08 pm
- Forum: DeBroglie Equation
- Topic: Post-Module Assessment #35 [ENDORSED]
- Replies: 2
- Views: 679
Re: Post-Module Assessment #35 [ENDORSED]
I think that only very very small objects have wavelike particles, and when they get too big, the wavelike properties are basically negligible, which is why they say electric cars would not have wavelike properties.
- Fri Oct 13, 2017 9:51 am
- Forum: Properties of Electrons
- Topic: Threshold level and Work Function [ENDORSED]
- Replies: 6
- Views: 1075
Re: Threshold level and Work Function [ENDORSED]
Yes they're the same thing
- Mon Oct 09, 2017 11:57 pm
- Forum: Properties of Light
- Topic: Converting to Different Units
- Replies: 3
- Views: 456
Re: Converting to Different Units
We will need to know what the prefixes mean so we can convert between them, like knowing that 10^12 picometers are in one meter. These conversions will not be given, so we have to remember what each prefix means. Hope this helps!
- Sat Oct 07, 2017 10:53 pm
- Forum: Significant Figures
- Topic: All students read this sig fig post [ENDORSED]
- Replies: 170
- Views: 34930
Re: All students read this sig fig post [ENDORSED]
In a multistep problem, should we use sig figs with every step, or only when it comes to the final answer? Will we be penalized if we use more than the amount of sig figs in intermediate steps but have the correct amount for the final answer? I am pretty sure we are supposed to only use sig figs in...
- Sat Oct 07, 2017 10:48 pm
- Forum: Empirical & Molecular Formulas
- Topic: Calculating ratios when finding molecular formulas [ENDORSED]
- Replies: 2
- Views: 418
Re: Calculating ratios when finding molecular formulas [ENDORSED]
It should come out to a whole number when you are trying to find out what to multiply the empirical formula by in order to get the molecular formula. Otherwise, there might have been a calculation error. Hope this helps! :)

