Search found 39 matches
- Thu Mar 08, 2018 12:06 am
- Forum: First Order Reactions
- Topic: Simpler way to calculate change in concentrations
- Replies: 2
- Views: 383
Re: Simpler way to calculate change in concentrations
No, you would not be penalized. That method is good and completely legitimate. I like it too. In fact, in the homework I noticed another trend in second order reactions that I do not believe would be penalized either, since it is just manipulating equations and finding trends. Here it is if I rememb...
- Thu Mar 08, 2018 12:00 am
- Forum: Second Order Reactions
- Topic: Second Order
- Replies: 3
- Views: 594
Re: Second Order
Both changes in concentration can be described by the half-life of a second order reaction: t_{1/2} = \frac{1}{k[A]_{0}} Since the initial concentration of A is in the denominator, the time it takes different initial concentrations to decay to half of their original concentration is dependent upon t...
- Wed Mar 07, 2018 11:52 pm
- Forum: General Rate Laws
- Topic: Coefficients
- Replies: 5
- Views: 591
Re: Coefficients
I believe that these coefficients do not affect how we write the rate laws for first and second order reactions. It would affect the unique rate of the reaction and finding the rates of consumption/production for one reactant/product in terms of another reactant/product's rate of consumption/product...
- Sat Mar 03, 2018 8:23 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3591758
Re: Post All Chemistry Jokes Here
What did C14 do for Valentine's Day?
He went carbon dating!
He went carbon dating!
- Sat Mar 03, 2018 3:52 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3591758
Re: Post All Chemistry Jokes Here
What do you do with a dead chemist?
Barium
Barium
- Sat Mar 03, 2018 3:48 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3591758
Re: Post All Chemistry Jokes Here
I don't trust atoms...
I heard they make up everything
I heard they make up everything
- Sat Feb 24, 2018 4:46 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Q of eqn [ENDORSED]
- Replies: 2
- Views: 429
Re: Q of eqn [ENDORSED]
As long as the reactants and products are not in the liquid or solid state, their concentrations and/or pressures affect the reaction quotient, Q, and the direction the reaction will proceed in to reach equilibrium. Both pressure and concentration can be included in the formula for the reaction quot...
- Sat Feb 24, 2018 4:42 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Rate constant (k)
- Replies: 4
- Views: 559
Re: Rate constant (k)
The rate constant K, is constant for a particular reaction at a particular temperature So yes, you're right; it may change with a temperature change, but usually these rate constants are measured by scientists in a lab setting, where temperature remains controlled. K is a very idealized constant bec...
- Sat Feb 24, 2018 4:35 pm
- Forum: General Rate Laws
- Topic: Reaction Rate and Spontaneity
- Replies: 5
- Views: 1500
Re: Reaction Rate and Spontaneity
I agree with both the other answers, spontaneity != reaction rate and vice versa
- Tue Feb 13, 2018 1:02 pm
- Forum: Calculating Work of Expansion
- Topic: Calculating work in Reversible and Irreversible Pathways
- Replies: 1
- Views: 307
Re: Calculating work in Reversible and Irreversible Pathways
-PdeltaV is for irreversible expansion and -nRTlnV2/V1 is for reversible expansion.
- Tue Feb 13, 2018 12:47 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Boltzmann Equation
- Replies: 2
- Views: 313
Re: Boltzmann Equation
It is important to specifically note that because when dealing with actual, complex molecules, it is difficult to know the exact number of microstates, W. It is often the case that we do not know the exact number of microstates, but that is okay because if we can estimate it, then we will still obta...
- Tue Feb 13, 2018 12:18 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Practice Midterm [ENDORSED]
- Replies: 5
- Views: 904
Re: Practice Midterm [ENDORSED]
Also if you search thoroughly enough on chemistry community there are past exam questions that students post about after they finished their exam, and even if they’re old they’re valuable to look at
- Sat Feb 10, 2018 9:40 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3591758
Re: Post All Chemistry Jokes Here
What is the show cesium and iodine were watching together?
CSI
CSI
- Sat Feb 10, 2018 9:38 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3591758
Re: Post All Chemistry Jokes Here
The first rule of thermodynamics is you don't talk about thermodynamics
- Sat Feb 10, 2018 9:37 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3591758
Re: Post All Chemistry Jokes Here
Thermodynamics:
First Law: You can't get anything without working for it.
Second Law: The most you can accomplish by work is to break even.
Third Law: You can't break even.
First Law: You can't get anything without working for it.
Second Law: The most you can accomplish by work is to break even.
Third Law: You can't break even.
- Tue Jan 23, 2018 8:14 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard Reaction Enthalpy units
- Replies: 1
- Views: 220
Re: Standard Reaction Enthalpy units
I believe the unit is kJ for the whole reaction because you cannot specify it to kJ/mol since each substance may have a different stoichiometric ratio, resulting in kJ/2mol, 2mol*kJ/mol... etc.
- Tue Jan 23, 2018 8:10 pm
- Forum: Administrative Questions and Class Announcements
- Topic: When can we pick up our finals from Chem 14A?
- Replies: 3
- Views: 798
Re: When can we pick up our finals from Chem 14A?
Final exams will be available 3rd week Winter quarter from 4006 Young Hall.
- Tue Jan 23, 2018 8:09 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.65
- Replies: 6
- Views: 711
Re: 8.65
They added the first reaction to half of the second reaction, and I am referring to the two reactions they provide in the problem.
Personally I would avoid dealing with fractions in these calculations if possible, and would not approach the problem like this.
Personally I would avoid dealing with fractions in these calculations if possible, and would not approach the problem like this.
- Sat Jan 20, 2018 6:25 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3591758
Re: Post All Chemistry Jokes Here
How do you tell the difference between a chemist and a train conductor?
Have them read "unionized"
Have them read "unionized"
- Sat Jan 20, 2018 5:30 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard reaction enthalpies
- Replies: 1
- Views: 439
Re: Standard reaction enthalpies
For this question, you can use the standard enthalpies of formation from Appendix 2A to calculate the standard reaction enthalpy, which is one of the methods to calculating a standard reaction enthalpy. Imagine that to carry out the reaction we convert the reactants into the elements in their most s...
- Sat Jan 20, 2018 10:45 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Problem 8.65
- Replies: 3
- Views: 414
Re: Problem 8.65
For your first question: It is because they preferred to write the chemical equation for forming 1 mole of N_{2}O_{5} first and then work their way towards that. You do not have to solve the problem this way, it is just how the textbook prefers to do it. For your second question: You actually can so...
- Sat Dec 02, 2017 11:34 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Link to Review Session Schedule? [ENDORSED]
- Replies: 3
- Views: 472
- Sat Dec 02, 2017 11:32 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: strength
- Replies: 2
- Views: 420
Re: strength
If it was two acids you could calculate the pH values and compare, whichever is lowest is a stronger acid. If it was two bases you could calculate the pH values and compare, whichever is higher is a stronger base. If it was an acid and a basic you could calculate the pH of the acid and the pOH of th...
- Sat Nov 25, 2017 11:18 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Molecular Shape Memorization
- Replies: 6
- Views: 1519
Re: Molecular Shape Memorization
I mean, unless you can memorize the basic shapes and then derive the rest by removing bonds and adding lone pairs of electrons and figure out the bond angle to maximize distance without memory, then yes it would have to simply be memorized.
- Sat Nov 25, 2017 11:10 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Expanded octet
- Replies: 6
- Views: 695
Re: Expanded octet
Also if an atom is at a lower energy, more stable state when an expanded octet is applied to one of the elements in the atom. So a quick lewis structure can double check if an element uses and expanded octet
- Sat Nov 11, 2017 9:55 pm
- Forum: Resonance Structures
- Topic: Resonance Structure vs. Isomer
- Replies: 1
- Views: 487
Re: Resonance Structure vs. Isomer
I believe the difference between a resonance structure and an isomer is resonance forms only differ in the arrangement of electrons while isomers differ in both the arrangement of atoms and electrons. As for chirality, I believe that is a special type of isomer and it is when two molecules are mirro...
- Sat Nov 11, 2017 9:43 pm
- Forum: Ionic & Covalent Bonds
- Topic: Covalent Bonding: Polar and Non-polar
- Replies: 6
- Views: 1058
Re: Covalent Bonding: Polar and Non-polar
Well, a polar covalent bond is a covalent bond between atoms that have partial electric charges. The chance of these partial electric charges occurring increases as the difference in the electronegativity between the atoms increases. An example is HCl. So two atoms in a covalent bond with a high ele...
- Sat Nov 04, 2017 8:17 pm
- Forum: Electronegativity
- Topic: ELECTRON AFFINITY
- Replies: 7
- Views: 1597
Re: ELECTRON AFFINITY
Also note that the atom wants to reach a stable, full valence shell, so whether that is easier by losing or gaining electrons will lead to a lower or higher electron affinity respectively.
- Sat Nov 04, 2017 8:12 pm
- Forum: Administrative Questions and Class Announcements
- Topic: 14B and 14BL
- Replies: 3
- Views: 426
Re: 14B and 14BL
14BL is titration and spectroscopy I heard, and I’ve also read it doesn’t matter either way.
- Sat Oct 28, 2017 11:59 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: 2.85
- Replies: 5
- Views: 505
Re: 2.85
Hello! If you remember the diagram Dr. Lavelle drew in lecture showing the different energy levels an electron in a Hyrdrogen atom can occupy, the n=1,2,3,... lines he drew correspond to the energy levels of the atomic orbitals of Hyrdogen with principal quantum numbers n=1,2,3,... Note that the ene...
- Sat Oct 28, 2017 9:09 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Nov 9th Midterm
- Replies: 1
- Views: 398
Re: Nov 9th Midterm
Hi! It seems they're already posted at https://lavelle.chem.ucla.edu/wp-content/supporting-files/Chem14A/Midterm_Review_Sessions_14A.pdf
Which is on the main 14A page at the link "Midterm Review Sessions and Rooms"
Which is on the main 14A page at the link "Midterm Review Sessions and Rooms"
- Thu Oct 19, 2017 12:19 pm
- Forum: Photoelectric Effect
- Topic: Finding the number of photons
- Replies: 2
- Views: 3018
Re: Finding the number of photons
Using E=h\upsilon and c=\lambda\upsilon you get: E=\frac{hc}{\lambda} Using that you can get the energy per photon of light emitted with the given wavelength of 1850 nm. Then: \frac{E_{total}}{E_{photon}} would give the number of photons generated in 1.0 s, since it is the energy emitted in 1.0 s di...
- Thu Oct 19, 2017 11:52 am
- Forum: Properties of Electrons
- Topic: 1.57
- Replies: 3
- Views: 470
Re: 1.57
For tests, should we know that 486 nm represents blue light, 700 nm is red, and so on? At office hours, Dr. Lavelle said we should know that roughly 400nm-700nm is visible light, 700 nm is red and 400 nm is violet and ultraviolet light is lower than 400nm and infrared light is higher than 700 nm. I...
- Wed Oct 18, 2017 5:29 pm
- Forum: DeBroglie Equation
- Topic: Homework 1.13/ Rydberg
- Replies: 3
- Views: 348
Re: Homework 1.13/ Rydberg
Personally, I would use the equation Dr. Lavelle used in class to avoid confusing myself:
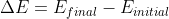
Where:
^{2}}+\frac{hR}{(n_{initial})^{2}})
That way I never have to deal with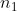 and
and 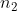
Where:
That way I never have to deal with
- Wed Oct 18, 2017 5:19 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: E-p distribution
- Replies: 2
- Views: 225
Re: E-p distribution
Since 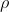 is rho, the electron density, I agree with the other reply saying he meant electron density distribution.
is rho, the electron density, I agree with the other reply saying he meant electron density distribution.
- Thu Oct 12, 2017 10:36 pm
- Forum: Properties of Light
- Topic: converting units ( keV )
- Replies: 2
- Views: 644
Re: converting units ( keV )
1 keV * \frac{1000eV}{1 keV} * \frac{1.602*10^{-19}C}{1e} eV stands for electron-volt. So in order to convert from keV, first you convert it to eV which is like any other unit with the kilo- prefix, then you multiply it by the charge of an electron. Couloumb (C) times Volt (V) makes a J so that sho...
- Thu Oct 12, 2017 10:22 pm
- Forum: Photoelectric Effect
- Topic: Constants that need to be memorized
- Replies: 6
- Views: 754
Re: Constants that need to be memorized
My TA asked Dr Lavelle and confirmed that the constants and equations will be provided for the test.
- Thu Oct 12, 2017 10:15 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Atomic Spectroscopy Post-Assessment Q28
- Replies: 2
- Views: 472
Re: Atomic Spectroscopy Post-Assessment Q28
In order to get the wavelength, you calculate 1 meter divided by 1,650,763.73 wavelengths:

The problem is saying there are 1,650,763.73 wavelengths in a meter, so the division simply obtains the very small portion of a meter that each wavelength is.
The problem is saying there are 1,650,763.73 wavelengths in a meter, so the division simply obtains the very small portion of a meter that each wavelength is.
- Thu Oct 05, 2017 3:27 am
- Forum: Molarity, Solutions, Dilutions
- Topic: G25 homework problem
- Replies: 7
- Views: 826
Re: G25 homework problem
I don't still don't really get how to do this problem. Can someone explain how to do it more? Well I started by reading the problem and seeing I have the molarity and the volume of the solution. So I can use the equation: M= \frac{n}{V} I want to find the moles of X in the solution, so I will switc...

