Search found 65 matches
- Sat Mar 17, 2018 12:52 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: 15.101A
- Replies: 3
- Views: 370
Re: 15.101A
I initially thought that Cl- should be included but the back of the textbook didn't include it and the question wasn't corrected in the Solution Manual Errors either, so I assumed that I was wrong. But if your answer book does include Cl- on the products then chemistry makes a lot more sense
- Sat Mar 17, 2018 1:50 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: confused about 2nd law !!! [ENDORSED]
- Replies: 3
- Views: 838
Re: confused about 2nd law !!! [ENDORSED]
The third law of thermodynamics is used to analyze the probability of finding one compound in a certain direction (think Schrodinger's cat) given the different orientations that it can have. The second law of thermodynamics will involve examining what will occur to the entropy of the system/surround...
- Sat Mar 17, 2018 1:45 am
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: 9.37 and subtracting vaporization
- Replies: 1
- Views: 419
Re: 9.37 and subtracting vaporization
The reactants are gases and the product is a liquid, hence, the entropy of the system increased
- Sat Mar 17, 2018 1:40 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: 15.101A
- Replies: 3
- Views: 370
Re: 15.101A
I believe this occurs because the reaction that produces Cl- is very slow, so that the reactions hardly forms an Cl- as products
- Sat Mar 17, 2018 1:38 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Entropy of Surroundings vs. System
- Replies: 2
- Views: 566
Re: Entropy of Surroundings vs. System
Hi, If a system is going from a solid state to liqud or liquid to vapor, then the entropy (disorder) of the system increases. Similarly, if a system is going from vapor to liquid or liquid to solid, then the entropy of the system decreases. Futhermore, because the entropy of the universe must always...
- Fri Mar 09, 2018 9:32 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Negative k? [ENDORSED]
- Replies: 5
- Views: 1083
Re: Negative k? [ENDORSED]
k cannot be negative, but there can be reactants with a negative sign in their reaction order
- Fri Mar 09, 2018 9:30 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: 15.65 c
- Replies: 2
- Views: 448
Re: 15.65 c
If the reaction is endothermic then increasing the temperature will favor the forward reaction; thus, k(1) will increase more than k'(1). (I do not have the textbook with me, so I might have answered the wrong question)
- Fri Mar 09, 2018 9:28 pm
- Forum: General Rate Laws
- Topic: Half Life
- Replies: 6
- Views: 794
Re: Half Life
Hi Diana! The half life tells you how fast/slow a compound decays. It is important because if you are storing a medicine, you might want to know how much is left after a certain time because if you are injecting medicine into a human you will want to know how much you are injecting and also how long...
- Thu Mar 08, 2018 10:58 am
- Forum: *Enzyme Kinetics
- Topic: Equation 18; Steric Factor
- Replies: 1
- Views: 339
Equation 18; Steric Factor
Do we have to memorize the equation 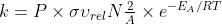 ? I could not find it on the constants and equations sheet
? I could not find it on the constants and equations sheet
- Sun Mar 04, 2018 12:32 pm
- Forum: General Rate Laws
- Topic: Half Life
- Replies: 3
- Views: 460
Re: Half Life
Yes, the half-life represents the same concept in all the rate laws. The half-life equation varies according to the order of the reaction because the integrated rate laws depend on the order of the reaction
- Sun Mar 04, 2018 12:27 pm
- Forum: General Rate Laws
- Topic: Differential and Integrated Rate Laws
- Replies: 3
- Views: 514
Re: Differential and Integrated Rate Laws
For our class most, if not all, problems will use the integrated rate law. The differential rate law is used to derive the integrated rate law
- Sun Mar 04, 2018 12:25 pm
- Forum: General Rate Laws
- Topic: half-life
- Replies: 8
- Views: 984
Re: half-life
Half-life can be used to approximate how much of a substance decayed or still remains. For instance, if you start with 100 mol of a substance with a half-life of 10 years then you can determine the following: 50% of the 100 mol, that is 50 moles, will have decayed in 10 years 50% of the 100 mol, tha...
- Sun Mar 04, 2018 12:21 pm
- Forum: General Rate Laws
- Topic: drivations
- Replies: 2
- Views: 448
Re: drivations
We are given the rate law equations on the equation sheet on the test and final, so I doubt that we will be asked to derive the equation. However, knowing how to derive the equations may increase your understanding of rate laws and would be nice to know in case we are asked to derived them. While I ...
- Sun Feb 25, 2018 2:19 pm
- Forum: General Rate Laws
- Topic: initial rate law
- Replies: 5
- Views: 612
Re: initial rate law
Because the rate law would become more complicated if we were to analyze the rxn when the presence of products is significant, we only consider the reaction at the very beginning when the amount of products can be considered negligible. While products also affect the rate law, we consider the rate l...
- Sun Feb 25, 2018 2:10 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: swapping signs of E values
- Replies: 8
- Views: 3698
Re: swapping signs of E values
You can also use the equation 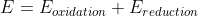
- Sun Feb 25, 2018 2:09 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Finding n
- Replies: 15
- Views: 1889
Re: Finding n
To find n, first balance the half reactions. Then multiple each half reaction with a coefficient that will allow you to cross out the moles of electrons on both half reactions. (i.e if one half reaction has 1 electron on the reactant side and the other half reaction has 4 electrons on the product si...
- Wed Feb 14, 2018 1:32 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 11.83
- Replies: 3
- Views: 1446
Re: 11.83
For 298K, you can use the equation  to find the K value. For 423K, use the equation
to find the K value. For 423K, use the equation =\Delta H/R [(1/T_{1})-(1/T_{2})])
- Sun Feb 11, 2018 11:13 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Gold Example
- Replies: 1
- Views: 258
Re: Gold Example
The example was mainly to proof why gold could be cleaned in a solution without gold dissolving, but if the object does not contain pure gold then there is a possibility that the object will dissolve.
- Sun Feb 11, 2018 11:06 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Midterm 2018W [ENDORSED]
- Replies: 4
- Views: 546
Midterm 2018W [ENDORSED]
Will there be lecturing on Wednesday 14 for Chem 14B?
- Sun Feb 11, 2018 11:05 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Maximum Potential
- Replies: 2
- Views: 784
Re: Maximum Potential
The maximum potential of a cell is the maximum voltage that can occur between the half reactions, but due to resistance and other factors the maximum cell potential is not typically reached
- Sun Feb 04, 2018 4:31 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Energy Free to do Useful Work
- Replies: 2
- Views: 414
Re: Energy Free to do Useful Work
Gibbs free energy measures the theoretical/maximum amount of work that a rxn could still do based on its "free" energy, however, because it is impossible for a reaction to be completely isolated, the "real" work that can be done will be less. I hope this clarifies the concept som...
- Sun Feb 04, 2018 3:41 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Delta G not
- Replies: 5
- Views: 792
Re: Delta G not
- Sun Feb 04, 2018 3:38 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Deriving Equations
- Replies: 2
- Views: 379
Deriving Equations
Will we need to know how to derive the equations for delta G? Or will the midterm be only on applying the equations?
- Sun Jan 28, 2018 11:27 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Open systems
- Replies: 6
- Views: 767
Re: Open systems
Because in reality isolated systems are difficult to engineer, I assume that we will be dealing with open and closed systems quite often
- Sun Jan 28, 2018 11:20 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Can a system exchange matter but not energy?
- Replies: 4
- Views: 471
Re: Can a system exchange matter but not energy?
I highly doubt this is possible because by exchange matter, one system would be losing the energy that is contained within the matter that was transferred
- Tue Jan 23, 2018 9:37 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Homework 8.49
- Replies: 4
- Views: 927
Re: Homework 8.49
Hence, we get the equation
R=3.145
T=298K
- Tue Jan 23, 2018 6:05 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: HW 8.41
- Replies: 3
- Views: 666
Re: HW 8.41
The reason that you continue to calculate 400.0g of water and not 450.0g of water is that you are considering them two separate systems. You do this because the 400.0g of water have a higher temperature than the 50.0g of water at every time interval except when the two systems finally reach equilibr...
- Sun Jan 21, 2018 8:46 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: W=-(Pex)(deltaV)
- Replies: 5
- Views: 1559
Re: W=-(Pex)(deltaV)
The negative sign is relative. Because we are calculating the work that the system performed on, say, a piston, the work represents the energy that was lost or gained by the system. If the final volume increased (expansion), then the system did work and, hence, the system lost energy If the final vo...
- Sun Jan 21, 2018 8:43 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Delta U?
- Replies: 7
- Views: 10646
Re: Delta U?
U is the internal energy of the system. Because the internal energy of a molecule would consist on the summation on the energies within atoms, bonds, etc (too complex to calculate), we use Delta U to discuss the change in the internal energy of a system without having to know the actual numerical va...
- Sun Jan 21, 2018 8:40 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Discussion 1J worksheet- Question 8
- Replies: 1
- Views: 538
Re: Discussion 1J worksheet- Question 8
Because the volume is held constant (Delta V=0), the gas cannot perform any work; thus, w=0 and delta U=q.
- Sun Jan 21, 2018 8:35 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Heat versus work
- Replies: 6
- Views: 830
Re: Heat versus work
Work is very similar to heat except that work has a direction (i.e energy is used to push a piston up) whereas heat is the random collision of fast and slow molecules, causing energy to be transferred from the faster moving molecules to the slower ones.
- Thu Jan 11, 2018 9:07 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Significant Figure [ENDORSED]
- Replies: 7
- Views: 1396
Re: Significant Figure [ENDORSED]
For rounding a number that ends in 5 (i.e 768.8465), round up iff the number preceeding 5 will become even and truncate iff the number preceeding 5 is already even
Ex. 0.98775 -> 0.9878
Ex. 0.98765 -> 0.9876
Ex. 0.98775 -> 0.9878
Ex. 0.98765 -> 0.9876
- Thu Jan 11, 2018 9:03 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 8.23
- Replies: 3
- Views: 441
Re: 8.23
Divide 22,500J by Delta T (23.97-22.45)Celcius to get J/C, which is the heat capacity of the calorimeter
- Thu Jan 11, 2018 8:58 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Liquids and Solids
- Replies: 3
- Views: 449
Re: Liquids and Solids
The back of the textbook should have a table of enthalpy values for the different physical states of common compounds, however, I dont think that there exist an equation that allows you to calculate the enthalpy of ice given the enthalpy of water
- Thu Jan 11, 2018 8:48 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Question 27
- Replies: 2
- Views: 243
Re: Question 27
For part a use the equation 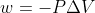 and for part b use equation w=-nRTln(V2/V1)
and for part b use equation w=-nRTln(V2/V1)
- Thu Dec 07, 2017 4:10 pm
- Forum: Lewis Acids & Bases
- Topic: strong or weak
- Replies: 3
- Views: 388
Re: strong or weak
As to tricks to distinguish strong versus weak acids/bases, I would suggest memorizing the strong acids and bases because they are only a few of them. Also, learn the trends that increase or decrease bond strength (i.e., number of Oxygen atoms, atomic radii, and electronegativity)
- Thu Dec 07, 2017 4:08 pm
- Forum: Lewis Acids & Bases
- Topic: strong or weak
- Replies: 3
- Views: 388
Re: strong or weak
When ions become more acidic (F- vs Cl-), HCl is more acidic because Cl has a bigger radius and hence the H-Cl bond strength decreases.
- Thu Dec 07, 2017 3:55 pm
- Forum: Lewis Acids & Bases
- Topic: Ch 12.115
- Replies: 1
- Views: 399
Ch 12.115
Acetic acid is use as a solvent for some reactions between acids and bases. a) Nitrous acid and carbonic acids are both weak acids in water. Will either of them act as a strong acid in acetic acid? Explain your answer. b) Will ammonia act as a strong or weak base in acetic acid? Explain your answer....
- Thu Dec 07, 2017 1:03 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: 12.49
- Replies: 5
- Views: 1344
Re: 12.49
You can predict that BrO- will be the stronger base. Because morphine has a N with a lone pair of electrons, H+ will bind to the nitrogen resulting in a very weak base. HBrO is a weak acid so its conjugate base BrO- will be relatively stronger.
- Sun Dec 03, 2017 10:40 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Concept Question
- Replies: 3
- Views: 555
Re: Concept Question
Although the answer that Dr. Lavelle said was by removing HN3, you could also change the volume since the reactants and products do not have the same amount of moles of gas (4 in reactants vs 2 in products). Thus, you could decrease the volume to increase the production of NH3
- Sun Dec 03, 2017 10:34 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Oxidation States
- Replies: 4
- Views: 4010
Oxidation States
How do you determine the oxidation state in a compound with a transition metal?
- Sat Nov 25, 2017 2:01 pm
- Forum: Lewis Acids & Bases
- Topic: Lewis Acid or Base: AlCl3 [ENDORSED]
- Replies: 3
- Views: 3882
Re: Lewis Acid or Base: AlCl3 [ENDORSED]
It readily accepts electrons from other atoms, in an attempt to get a full valence shell of eight electrons, thus it behaves as a Lewis acid since it is an electron acceptor
- Sat Nov 25, 2017 1:57 pm
- Forum: Lewis Acids & Bases
- Topic: Determining the Lewis Acid/Base
- Replies: 2
- Views: 419
Re: Determining the Lewis Acid/Base
The Lewis acid is the reactant that can accept an electron from the Lewis base, which can donate an electron
- Sat Nov 18, 2017 2:10 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Radicals
- Replies: 4
- Views: 635
Re: Radicals
I calculate the formal charges on every atom in the compound and if one atom has a +1 formal charge (assuming all other atoms have a formal charge of zero), then I would place the radical electron on the atom with the +1 formal charge so that the new formal charge will be 0 and the molecule will be ...
- Sat Nov 18, 2017 2:05 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bent or linear?
- Replies: 11
- Views: 2024
Re: Bent or linear?
I believe the shape would depend on where the lone pairs of electrons are placed and not be affected by single or double bonds
- Sun Nov 12, 2017 7:22 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Radicals
- Replies: 2
- Views: 405
Re: Radicals
Elements with radical electrons are also more reactive and do not undergo rearrangement to produce a more stable form (I believe they steal electrons from other elements instead)
- Sun Nov 12, 2017 7:17 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Formal charge and lewis structures
- Replies: 5
- Views: 611
Re: Formal charge and lewis structures
If you are asked to draw the most stable Lewis structure then, I believe, you can only draw one Lewis structure if the one you drew is the most stable. However, if you have difficult drawing the most stable Lewis structure I would suggest drawing multiple structures to then reason your way into draw...
- Thu Nov 09, 2017 4:01 pm
- Forum: Bond Lengths & Energies
- Topic: dissociatioin energies
- Replies: 3
- Views: 436
Re: dissociatioin energies
N has a half-full shell (p^3) so it has a lower electronegativity than C because adding another electron to N would increase e-e repulsion; therefore, CH has a higher disassociation energy than NH
- Fri Nov 03, 2017 5:46 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Heisenberg Concept Question
- Replies: 2
- Views: 353
Re: Heisenberg Concept Question
Because an electron is extremely small, if you measure its position by using light (a photon), the photon will collide with the electron and thus, you will know its velocity but the position of the electron will not be certain because it may have had change direction after the measurement of its vel...
- Fri Nov 03, 2017 5:25 pm
- Forum: Electronegativity
- Topic: CO2 vs. CS2 ionic character
- Replies: 3
- Views: 3925
Re: CO2 vs. CS2 ionic character
I don't believe that you need to know that sulfur has a higher electronegativity than carbon (if you search up a periodic table that shows electronegativity, you can see that S and C have roughly the same electronegativity). To measure which compound is more ionic you compare O and S since Carbon is...
- Fri Oct 27, 2017 10:27 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Highest precision in velocity
- Replies: 2
- Views: 582
Re: Highest precision in velocity
Because of the uncertainty principle equation, 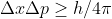 ,
, 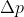 is proportional to
is proportional to )
Hence, if Delta X increases than Delta p decreases and p=mv so velocity will also decrease
Hence, if Delta X increases than Delta p decreases and p=mv so velocity will also decrease
- Fri Oct 27, 2017 10:22 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Chapter 1 #43 [ENDORSED]
- Replies: 7
- Views: 936
Re: Chapter 1 #43 [ENDORSED]
Alissa, I believe the 2pi comes from derivation of the formula that is outside the scope of the course. However, you may prefer to read "Uncertainty Principle Equation" in wikipedia for more information about how the equation was derived.
- Fri Oct 27, 2017 10:13 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: 1. 44
- Replies: 1
- Views: 351
Re: 1. 44
Delta v= 5.0 m/s and the mass of a hydrogen atom is (1.0079x10^-3)/(6.022x10^23) kg/atom
- Fri Oct 27, 2017 10:08 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Angular Variables
- Replies: 3
- Views: 471
Re: Angular Variables
I also thought that angular momentum should have some level of uncertainty, but I believe it does not because the problem states that angular momentum has definite qunatized values. However, the equation would not make sense since h would be divided by 0.
- Sat Oct 21, 2017 1:27 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: ch1 question 43
- Replies: 8
- Views: 1139
Re: ch1 question 43
h-bar represents 
(Planck's constant divided by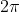 )
)
(Planck's constant divided by
- Sat Oct 21, 2017 1:23 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Chapter 1 #43 [ENDORSED]
- Replies: 7
- Views: 936
Re: Chapter 1 #43 [ENDORSED]
* 6.626x10^-34 Js
- Sat Oct 21, 2017 1:23 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Chapter 1 #43 [ENDORSED]
- Replies: 7
- Views: 936
Re: Chapter 1 #43 [ENDORSED]
The value for h is still ^-^3^4)
Js, but in Heisenberg's Indeterminacy Equation which is equal to
which is equal to
1.054x^-^3^4)
Js, but in Heisenberg's Indeterminacy Equation
1.054x
- Sat Oct 21, 2017 1:14 pm
- Forum: DeBroglie Equation
- Topic: How do we get E=pv? [ENDORSED]
- Replies: 2
- Views: 558
Re: How do we get E=pv? [ENDORSED]
- Wed Oct 18, 2017 7:04 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Angular Variables
- Replies: 3
- Views: 471
Re: Angular Variables
I manage to convert the equation in the textbook, delta X and delta momentum > h/4pi into )
but I don't know how to interpret this equation given that angular momentum has no uncertainty.
but I don't know how to interpret this equation given that angular momentum has no uncertainty.
- Wed Oct 18, 2017 6:59 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Angular Variables
- Replies: 3
- Views: 471
Angular Variables
We can state Heisenberg's Uncertainty Principle in terms of angular variable as \Delta L \Delta \phi \geq h , where delta L is the uncertainty in angular momentum and delta phi is the uncertainty in angular position. For electrons in an atom, the angular momentum has definite quantized values with n...
- Wed Oct 18, 2017 10:14 am
- Forum: Student Social/Study Group
- Topic: Rieber Vista(Or any of the Riebers') Chem 14A Study Group Fall 2017
- Replies: 6
- Views: 1137
Re: Rieber Vista(Or any of the Riebers') Chem 14A Study Group Fall 2017
Hey Sharon
I am at Rieber Hall and I would like to join your study group! My email is diego.zavala2017@gmail.com
I am at Rieber Hall and I would like to join your study group! My email is diego.zavala2017@gmail.com
- Sat Oct 14, 2017 11:45 pm
- Forum: DeBroglie Equation
- Topic: Deriving DeBroglie Equation
- Replies: 2
- Views: 535
Re: Deriving DeBroglie Equation
E=mc^2 & E=hf; therefore,
mc^2=hf (f=frequency)
mc^2=h(c/y) (y=lambda)
mc=h/y
mv=h/y Since in E=mc^2, c represents the speed of light we can then replace c with velocity and it follows that:
y=h/(mv)
y=h/p
mc^2=hf (f=frequency)
mc^2=h(c/y) (y=lambda)
mc=h/y
mv=h/y Since in E=mc^2, c represents the speed of light we can then replace c with velocity and it follows that:
y=h/(mv)
y=h/p
- Sat Oct 14, 2017 11:39 pm
- Forum: DeBroglie Equation
- Topic: chapter 1 , question 23
- Replies: 2
- Views: 621
Re: chapter 1 , question 23
You will also need the conversion factor of 1.602x10^-19J/eV (don't forget to convert kJ to joules)
- Sat Oct 07, 2017 10:48 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Rydberg's Equation [ENDORSED]
- Replies: 2
- Views: 612
Re: Rydberg's Equation [ENDORSED]
Because E=hvand E=hR[z], the first equation can be rewritten as v=R[z]. Also because (Lambda)x(frequency)=c, Bohr's equation can be written as c/(Lambda)=R[z].
z=(1/n(1)^2)-(1/n(2)^2).
z=(1/n(1)^2)-(1/n(2)^2).
- Sat Oct 07, 2017 10:35 pm
- Forum: *Shrodinger Equation
- Topic: Example from Summer Test #1, Question 4a [ENDORSED]
- Replies: 2
- Views: 601
Re: Example from Summer Test #1, Question 4a [ENDORSED]
Because v(frequency)=R[(1/n(1)^2)-(1/n(2)^2)], so v=R[(1/(1)^2)-(1/(3)^2)] and, if multiplied by -h the equation can be rewritten as E=-hR[(1/3^2)-(1/1^2)].

