Search found 59 matches
- Wed Mar 14, 2018 12:27 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: 15.61 and Equation used to solve it
- Replies: 3
- Views: 538
Re: 15.61 and Equation used to solve it
15.61 states: "The rate constant of the rst-order reaction 2 N2O(g) + S --> 2 N2(g) + O2(g) is 0.76 s 1 at 1000. K and 0.87 s 1 at 1030. K. Recall that the rate constant changes with different temperatures. We are still doing the same reaction, but simply at different temperatures with differen...
- Tue Mar 13, 2018 11:55 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Chapter 15.12?
- Replies: 4
- Views: 473
Re: Chapter 15.12?
The equations are basically a mathematical definition of A (collision frequency) and a more complex version of ln(k)=\frac{E_{a}}{RT} + ln(A) . I am 99% that you only be using the this equation and not the one that they give you in section 15.12. As for what you should know from the ...
- Tue Mar 13, 2018 11:38 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: 15. 85
- Replies: 1
- Views: 301
Re: 15. 85
First you're finding the balanced equation. After that, look at the number of different reactants of the balanced equation. This will determine the molecularity. 1 reactant is unimolecular, 2 is bimolecular, 3 termolecular. Finally, you draw the activated complex, which will form at the top of the a...
- Sat Mar 10, 2018 12:32 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Microscopic Reversibility [ENDORSED]
- Replies: 1
- Views: 283
Re: Microscopic Reversibility [ENDORSED]
Of course, it is very possible for the forward and reverse reactions to follow different pathways, especially since the reverse reaction will face a higher energy barrier and possibly be an endergonic reaction (assuming that the forward is favorable). In this case, the intermediate could react anyth...
- Sat Mar 10, 2018 12:25 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: HW 15.47
- Replies: 2
- Views: 301
Re: HW 15.47
I also find it helpful to redraw the steps using numbers or letters corresponding to each different molecule. It makes it a bit simpler to see than that complicated mess.
- Sat Mar 10, 2018 12:21 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Transition States [ENDORSED]
- Replies: 2
- Views: 304
Re: Transition States [ENDORSED]
If you picture the reaction profile, the transition state is that hump between the products and reactants. Because the transition state is higher on the graph, it indicates that it is higher in energy.
- Fri Mar 02, 2018 10:04 pm
- Forum: Balancing Redox Reactions
- Topic: Inert Conductors [ENDORSED]
- Replies: 2
- Views: 484
Re: Inert Conductors [ENDORSED]
Are there other inert conductors, other than platinum that we should familiarize ourselves with for this class? Not really. Platinum is usually the go-to one, at least for this course. That being said, remember that other solid metals can serve as conductors too, along with the special case of liqu...
- Thu Mar 01, 2018 10:42 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Order of Cell Diagrams [ENDORSED]
- Replies: 1
- Views: 261
Re: Order of Cell Diagrams [ENDORSED]
In cell diagrams, how do we order the compounds or molecules on one of the sides of the salt bridge. For example, for the anode, are we supposed to order the electrodes in a specific order? Like by alphabetical order of states or by reactants then products? I'm not sure this question made any sense...
- Thu Mar 01, 2018 10:39 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Example 14.4 [ENDORSED]
- Replies: 1
- Views: 285
Re: Example 14.4 [ENDORSED]
The H+ and Cl- are implied in the HCl(aq). They just chose to combine them instead of writing them both out separately.
- Wed Feb 21, 2018 11:47 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: 14.91 Electrolyte
- Replies: 2
- Views: 437
Re: 14.91 Electrolyte
i think this is referring to the external wire circuit. From what i know, salt bridges don't exist in Electrolytic cells. It mentioned the external wire circuit in the problem so I don't think it's that. The problem wants to know how the "current is carried through the cell itself." What ...
- Mon Feb 19, 2018 10:46 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell reactions
- Replies: 1
- Views: 230
Re: Cell reactions
In problem 13, part d, how do we know which side is the cathode and which side is the anode? When you write out your half reactions, write them both with e- added. Then when you look at your given skeleton equation, figure out which half reaction you need to flip in order to get the equation. That ...
- Mon Feb 19, 2018 10:43 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 14.21 and Cell Diagrams/Standard Potentials
- Replies: 1
- Views: 237
Re: 14.21 and Cell Diagrams/Standard Potentials
How come for both of the half reactions derived from the cell diagram, you add the electron in both cases? Shouldn't the left side of the cell diagram be an oxidation reaction, in which the species loses an electron instead of gaining an electron? Yes, but they're usually written with the electron ...
- Mon Feb 19, 2018 10:39 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: 14.33 (b) Half-reactions
- Replies: 2
- Views: 457
14.33 (b) Half-reactions
14.33 (b): Will Tl+ disproportionate in aqueous solution? The solution manual uses the half equations Tl^{+} + e^{-} \rightarrow Tl and Tl^{+} \rightarrow Tl^{3+} + 2e^{-} . Why can't you use Tl \rightarrow Tl^{3+} + 3e^{-} instead of Tl^{+} \rightarrow Tl^{3+} + 2e^{-} ? Both would get you the equa...
- Tue Feb 13, 2018 11:56 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Standard Molar Entropy Question
- Replies: 3
- Views: 671
Re: Standard Molar Entropy Question
Helen Shi 1J wrote:So the complexity of the molecule overrules positional disorder?
I think so. As far as my memory goes, I've only seen positional disorder come to play when T=0K.
- Tue Feb 13, 2018 11:47 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Standard Gibbs Free Energy doesn't change?
- Replies: 1
- Views: 281
Re: Standard Gibbs Free Energy doesn't change?
Does standard Gibbs free energy stay the same for the molecule at whatever condition? I thought it changed based on temperature due to the equation delta G standard = delta H standard - T*delta S standard. So when you plug in any temperature, you get a delta G standard based on the temperature. How...
- Tue Feb 13, 2018 11:45 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Using the specific heat capacity ice
- Replies: 1
- Views: 402
Re: Using the specific heat capacity ice
4.184 J/(C*g) is the heat capacity for water when it is in the liquid state. The specific heat capacity of ice functions exactly like the Cp for liquid water only it is for when the water is a solid.
- Thu Feb 08, 2018 10:52 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.73
- Replies: 1
- Views: 283
Re: 8.73
How do you know if you need to break a bond or not? For example in 8.73 you didnt need to break the c-h This is much easier to see if you draw out both the reactants and products. I compare the two structures and see which ones I have to break and form. As for why you didn't need to break the C-H b...
- Thu Feb 08, 2018 10:37 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: textbook problem 9.25
- Replies: 1
- Views: 329
Re: textbook problem 9.25
9.25 If SO2F2 adopts a positionally disordered arrangement in its crystal form, what might its residual molar entropy be? I am not sure how to approach this problem. Can someone please explain to me the difference between "positionally disordered arrangement" and "residual entropy&qu...
- Thu Feb 08, 2018 10:29 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 9.69
- Replies: 1
- Views: 265
Re: 9.69
For this problem, why isn't the free energy of the reaction converting ADP to ATP added in along with reactions 2 and 3? The ATP reaction requires energy, which means that to produce ATP, the reaction needs to be coupled with another reaction that gives off energy. Reactions 2 and 3 both give off f...
- Thu Feb 01, 2018 9:18 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Problem 9.13
- Replies: 4
- Views: 592
Re: Problem 9.13
For problem 9.13, why is R used instead of heat capacity for the entropy equation regarding temperature change? Heat capacity by definition is the amount of energy required to raise the temperature of a certain amount of substance by 1 kelvin. In the problem however, you're not raising or lowering ...
- Thu Feb 01, 2018 9:09 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Homework 9.21
- Replies: 3
- Views: 431
Re: Homework 9.21
Adding on to that, for this specific problem the number of states will be the number of orientations. In a, all of the molecules face the same way and therefore have only one possible orientation. In b, you will have four orientations so w = ln(4^# of molecules).
- Thu Feb 01, 2018 9:05 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: 9.1
- Replies: 1
- Views: 263
Re: 9.1
In part a in question 9.1, the solution manual puts a negative sign in front of the rate of heat generation then drops it in the final answer. Is this an error in the manual? I think it might be because the question is asking for only the rate or the magnitude at which the body generates entropy in...
- Wed Jan 24, 2018 11:10 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Calculating heat (q)
- Replies: 4
- Views: 2149
Re: Calculating heat (q)
How do you know when to use q=mc(delta)T vs when to use q=nc(delta)T? This all depends on whether you have the specific heat capacity (Csp) or the molar heat capacity (Cm). If you have the specific heat capacity, use q=m*Csp*deltaT. If you have the molar heat capacity, use q=n*Cm*delta T. Note that...
- Wed Jan 24, 2018 10:52 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Internal Energy
- Replies: 5
- Views: 525
Re: Internal Energy
William Lan 2l wrote:If delta P = 0 (constant pressure), why is delta U = q + w? I thought that w = -PdeltaV. If P is 0, there would be no work then right?
In w = -P * delta V, you would plug in the constant pressure for P, not the change in P which would be delta P.
- Wed Jan 24, 2018 10:50 pm
- Forum: Phase Changes & Related Calculations
- Topic: Chapter 8 Homework #99
- Replies: 2
- Views: 511
Re: Chapter 8 Homework #99
Because ZnCl2 is aqueous, just treat them as Zn2+ and 2Cl-.
- Mon Jan 15, 2018 1:27 pm
- Forum: Calculating Work of Expansion
- Topic: Constant External Pressure
- Replies: 2
- Views: 271
Re: Constant External Pressure
One of the equations we are given for work is w=-PexΔV, where external pressure is constant. Will the pressure always be constant or are there instances where the pressure will be different? For that equation to be used, the pressure has to be constant. However, if the pressure is not constant, the...
- Mon Jan 15, 2018 1:08 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: 8.7
- Replies: 2
- Views: 347
Re: 8.7
Why is 982 delta U? Wouldn’t it be 982J- 492J? Aren’t work and delta U the same thing as according to 8.3 c? The internal energy of a system is delta U, so delta U is given at 982 J. The equation is delta U = w + q, where heat is given at +492 J, so 982J - 492J will give you how much work is done. ...
- Mon Jan 15, 2018 1:00 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Homework Problem 8.31
- Replies: 2
- Views: 181
Re: Homework Problem 8.31
It looks that way. I would memorize the values for the heat capacity at constant volume and add R to that value to get at constant pressure.
- Wed Jan 10, 2018 9:52 pm
- Forum: Calculating Work of Expansion
- Topic: Example 8.2: Calculating the work in isothermal expansion
- Replies: 1
- Views: 243
Re: Example 8.2: Calculating the work in isothermal expansion
Given in this example: A piston confines 0.100 mol Ar(g) in 1.00L at 25 degrees Celsius. a) The gas is allowed to expand through an additional 1.00L against a constant pressure of 1.00 atm. The book says to use Equation 3 (w= -P ex \Delta V ) to part a because it is an "irreversible path"...
- Wed Jan 10, 2018 8:52 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Systems
- Replies: 3
- Views: 347
Re: Systems
Open system - matter in a system are free to interact with the surrounding environment. Ex: Breathing in and out air Closed system - matter in a system is separated from matter in the environment but heat/energy can still be exchanged. Ex: Closed water bottle. Isolated system - neither matter nor en...
- Wed Jan 10, 2018 8:23 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Method 2 for Calculating Net Enthalpy
- Replies: 2
- Views: 165
Re: Method 2 for Calculating Net Enthalpy
When calculating net enthalpy using Method 2 (not Hess's Law), are the bonds of the reactants always broken and the bonds of the products always formed? Or can the bonds of the products be broken and the bonds of the reactants be formed? If you're breaking bonds in the products and forming bonds in...
- Wed Jan 10, 2018 8:07 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Why is fusion another name for melting?
- Replies: 4
- Views: 460
Re: Why is fusion another name for melting?
You could also think about it in terms of the intermolecular bonds. In the gas phase, the molecules are too far apart to have any effect on each other but in the liquid phase, the molecules form intermolecular bonds that more or less hold the molecules together, thereby "fusing" them in a ...
- Thu Dec 07, 2017 3:22 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 4.19 c)
- Replies: 1
- Views: 385
Re: 4.19 c)
I think the crux of the problem here is that you are assuming that the added electron doesn't pair with the lone electron on B. The reason why most atoms tend to want 4 bonds is so it can fulfill its octet, but remember, boron is one of those weird ones that do not need their octets filled. Instead,...
- Thu Dec 07, 2017 3:14 pm
- Forum: Lewis Acids & Bases
- Topic: Bronsted vs. Lewis
- Replies: 6
- Views: 992
Re: Bronsted vs. Lewis
Also, all Bronsted acids are Lewis acids and all Bronsted bases are Lewis acids. The Bronste acids and bases are just a special case of Lewis acids and bases.
- Wed Nov 29, 2017 3:25 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Order of ligands [ENDORSED]
- Replies: 2
- Views: 406
Re: Order of ligands [ENDORSED]
You will still keep them in alphabetical order for the formula.
- Wed Nov 29, 2017 3:23 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Calculating the Equilibrium Constant with Coefficients
- Replies: 2
- Views: 456
Re: Calculating the Equilibrium Constant with Coefficients
Why do you raise each species in the equilibrium constant to its coefficient because when doing an ICE table you already add/subtract the change by the coefficient times x? For example, for 3H2 + N2 --> 2NH3, why do you raise the coefficent to each species after you already subtract or add by -3x o...
- Thu Nov 23, 2017 1:55 pm
- Forum: Naming
- Topic: H2O in coordination compounds
- Replies: 2
- Views: 403
Re: H2O in coordination compounds
No because they're the same molecule, water. The only difference is that OH2 emphasizes that it is O that does the bonding.
- Wed Nov 22, 2017 4:18 pm
- Forum: Ideal Gases
- Topic: Reverse Reaction, Q and K
- Replies: 5
- Views: 1023
Re: Reverse Reaction, Q and K
When Q > K, that means that you start with the product first, and then the reaction will start to occur to create more reactants. However, the reaction will never create more reactants than is required for the equilibrium. That's what Dr. Lavelle meant by "not overshooting the equilibrium"...
- Thu Nov 16, 2017 10:40 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: bond angle
- Replies: 4
- Views: 2273
Re: bond angle
Why do we say bonds are less than 109 degrees or less than 120 degrees? I don't understand why it is "less than" Lone pairs exert more repelling force against bonded pairs than do bonded against bonded. So if we know that a molecule is usually 120 degrees with all three electron densities...
- Thu Nov 16, 2017 10:35 pm
- Forum: Hybridization
- Topic: Orientation of Hybrid Orbitals: 4.31
- Replies: 2
- Views: 532
Re: Orientation of Hybrid Orbitals: 4.31
They are just like when you are figuring out the shape of the molecules, only you don't exactly call them "tetrahedrons" or "trigonal planners" and such. Instead, you say that the orbitals are oriented towards the corners of [insert shape] (with the exception of the linear shape,...
- Wed Nov 15, 2017 6:23 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Understanding sigma and pi bonds?
- Replies: 8
- Views: 852
Re: Understanding sigma and pi bonds?
Also, sigma bonds are formed by hybridized orbitals overlapping. Unhybridized orbitals formal pi bonds.
- Sun Nov 12, 2017 11:46 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: AXE notation [ENDORSED]
- Replies: 3
- Views: 1795
Re: AXE notation [ENDORSED]
A - Means atom. You will always have this. X - Number of bonded pairs to the central atom. You will have at least on X in order for there to be a molecule. E - Number of lone pairs on the central atom. It is possible to not have any lone pairs, so you will simply omit E if needed. The number of bond...
- Sun Nov 12, 2017 11:43 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lone Pair Placement
- Replies: 3
- Views: 450
Re: Lone Pair Placement
Starting with the base model where both bonds and lone pairs are included, you place the lone pairs where it will affect the least number of bonded pairs at the lowest angle. For example, let's say you first have a trigonal bipyramidal model and are trying where to place the lone pairs. You would pl...
- Tue Nov 07, 2017 4:37 pm
- Forum: Lewis Structures
- Topic: Exceptions to octet rule?
- Replies: 6
- Views: 1449
Re: Exceptions to octet rule?
In addition to Be and B like you mentioned, I wanted to note that H and He also never follow the octet rule because they are more than happy only filling the 1s orbitals, which is the only type of orbitals the n=1 energy level.
- Tue Nov 07, 2017 4:32 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Configuration of Ions
- Replies: 2
- Views: 330
Re: Electron Configuration of Ions
I think it might have something to do with there being only 1 electron in the p orbital. The elements in Group 13 are more likely to give up electrons to empty the 1 electron in the p orbitals and then 2 in the s orbitals than to gain 5 electrons to fill p.
- Sun Nov 05, 2017 9:30 pm
- Forum: Lewis Structures
- Topic: 11/5 ch3 review
- Replies: 2
- Views: 358
Re: 11/5 ch3 review
I think it was because O is more electronegative than C, and therefore H is more attracted to O than to C. Also, if H were to bond with one of the C, then that C atom cannot form any double bonds with O. When you try to calculate the formal charges, you'll see that this molecule will yield a net cha...
- Mon Oct 30, 2017 11:21 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: electron configuration of ions, q 3.5 [ENDORSED]
- Replies: 2
- Views: 299
Re: electron configuration of ions, q 3.5 [ENDORSED]
can anybody explain why according to the solutions manual, in question 3.5 the ground-state electron configuration from Ga^3+ is [Ar]3d^10 instead of [Ar]3d^8 4s^2, and also why the ground-state configuration for Tl^3+ is [Xe]4f^14 5d^10 instead of [Xe]4f^14 5d^8 6s^2? The ground state for the neut...
- Mon Oct 30, 2017 8:37 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Heisenberg Indeterminacy (Uncertainty) Equation
- Replies: 2
- Views: 783
Re: Heisenberg Indeterminacy (Uncertainty) Equation
The hydrogen atom has a radius of approximately 0.05 nm. Assume that we know the position of an electron to an accuracy of 1 % of the hydrogen radius, calculate the uncertainty in the speed of the electron using the Heisenberg uncertainty principle. Comment on your value obtained. How would we dete...
- Sat Oct 28, 2017 3:06 pm
- Forum: Hybridization
- Topic: Principle Quantum Number in D-Block [ENDORSED]
- Replies: 4
- Views: 1067
Re: Principle Quantum Number in D-Block [ENDORSED]
The d-block actually belongs to the shell above, but because the energy difference between each principal level gets smaller and smaller as n increases, the electrons start to fill in the ns orbitals before they start filling in the (n-1)d orbitals. (I like to think of it as the electrons getting co...
- Sat Oct 28, 2017 2:58 pm
- Forum: Significant Figures
- Topic: 300 vs 300. [ENDORSED]
- Replies: 8
- Views: 4751
Re: 300 vs 300. [ENDORSED]
Yup! :)
- Wed Oct 25, 2017 10:17 pm
- Forum: Lewis Structures
- Topic: Lewis Dot Structure Example
- Replies: 3
- Views: 989
Re: Lewis Dot Structure Example
NH4+ and SO4- are held together by ionic bonds. The Lewis dot diagrams represent covalent bonding so you can't use it to represent the entire ionic compound.
- Wed Oct 25, 2017 9:52 pm
- Forum: Lewis Structures
- Topic: Lewis Dot Structure
- Replies: 2
- Views: 294
Re: Lewis Dot Structure
You don't have to, but I usually do it anyway so I can keep track of how many unpaired valence electrons I have and how many I need.
- Mon Oct 23, 2017 7:12 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Core Electron Question
- Replies: 2
- Views: 397
Re: Core Electron Question
To add on to that, by definition core electrons do not include any valence electrons. The noble gases have all of their orbits filled in their highest principle quantum level and therefore contain only core electrons. These electrons are almost guaranteed to be stable and nonreactive, so you don't h...
- Sat Oct 21, 2017 2:31 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: orbitals an shells [ENDORSED]
- Replies: 5
- Views: 1129
Re: orbitals an shells [ENDORSED]
Can you clarify the difference between shells and subshells? Shells correspond to the principle quantum number (n). Within these shells are the different types of subshells (s, p, d, f), which are the angular momentum quantum number (l). So for example, the orbitals 2s and 2p are within the same sh...
- Mon Oct 16, 2017 9:35 pm
- Forum: Photoelectric Effect
- Topic: Number of Photons
- Replies: 4
- Views: 447
Re: Number of Photons
Yes, the greater the intensity, the more photons you'll have. Since theoretically, each photon ejects one electron given that it has enough energy, the number of electrons ejected will increase too. This is based on the assumption that the photon already has enough energy to eject an electron. If th...
- Mon Oct 16, 2017 9:29 pm
- Forum: Photoelectric Effect
- Topic: Hey I stumbled upon these questions. Please help!
- Replies: 4
- Views: 1105
Re: Hey I stumbled upon these questions. Please help!
21. According to the wave model, a greater intensity would result in more energy, but this still did not eject the electrons. This was surprising because it meant stripping the wave model and viewing the rays as photons, which requires you to increase the frequency/decrease the wavelength to increas...
- Tue Oct 10, 2017 3:23 pm
- Forum: Properties of Light
- Topic: Atomic Spectra
- Replies: 1
- Views: 237
Re: Atomic Spectra
You won't get a negative value because the value for n_{1} (initial) is always going to be lower than n_{2} (final). When you put those values under a fraction, the previously larger value will become the smaller one, and vice versa. Let's use your example of 1 and 5: v=R(\frac{1}{n_{1}^{2}}-\fr...
- Tue Oct 10, 2017 3:09 pm
- Forum: Properties of Light
- Topic: Problem 1.4
- Replies: 3
- Views: 501
Re: Problem 1.4
I can't see a correct answer either. Maybe the book is trying to pull one over us, and there is none???
- Tue Oct 10, 2017 2:59 pm
- Forum: Properties of Light
- Topic: Amplitude and Frequency
- Replies: 1
- Views: 228
Amplitude and Frequency
I understand the inverse relationship between the wavelength and frequency, 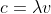 , and I know that amplitude apparently does not affect this relationship. But why? Wouldn't the amplitude also affect the frequency because of the extra distance traveling up and down the wave with a fixed speed?
, and I know that amplitude apparently does not affect this relationship. But why? Wouldn't the amplitude also affect the frequency because of the extra distance traveling up and down the wave with a fixed speed?

