Search found 50 matches
- Tue Mar 13, 2018 10:25 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Rate Determining Step
- Replies: 2
- Views: 361
Re: Rate Determining Step
I think the rate determining step would be the slowest of the slow steps overall.
- Tue Mar 13, 2018 10:23 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Catalysts
- Replies: 2
- Views: 370
Re: Catalysts
Catalysts increase the k value in the rate law by lowering the activation energy of the reaction. They do not show up when writing the overall reaction and are not directly included in the rate law, only indirectly by changing the k value.
- Tue Mar 13, 2018 10:18 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Adsorption
- Replies: 4
- Views: 675
Re: Adsorption
Adsorption is when the catalyst is a solid and the reaction takes place on its surface. This image is an example to help visualize: 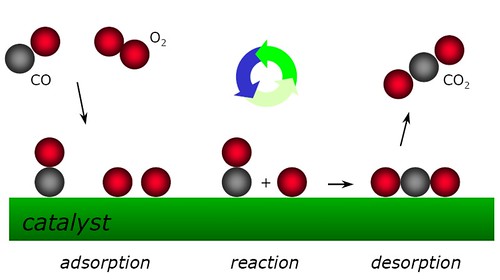

- Sun Mar 11, 2018 9:52 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: 15.71
- Replies: 2
- Views: 286
Re: 15.71
H2O cannot be an intermediate because it is one of the reactants in the overall reaction.
- Sun Mar 11, 2018 9:50 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: 15.65 (c)
- Replies: 2
- Views: 396
Re: 15.65 (c)
This can be seen in the Arrhenius equation: ln(k)=-Ea/RT+ln(A) where Ea is the activation energy. The slope of the line plotted on a ln(k) vs 1/T graph is -Ea/R. This means that for higher activation energies, the slope is more steeply negative so the ln(k) value, and also the k value, changes more ...
- Sun Mar 11, 2018 9:18 pm
- Forum: First Order Reactions
- Topic: Pseudo First Order Reaction
- Replies: 7
- Views: 1735
Re: Pseudo First Order Reaction
We use pseudo first order reactions when we are studying a reaction with multiple reactants with concentrations that could affect the rate law. For example if the reaction in question is A+B-->P we would design an experiment to find out how the rate changes with the concentrations of each reactant. ...
- Fri Mar 02, 2018 12:07 am
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Straight line
- Replies: 7
- Views: 1766
Re: Straight line
Yes, the straight line is a best fit line to the data. When trying to determine the order of a reaction from the data, you need to plot the data and see what operation you need to do to make the best fit curve be a straight line instead of an exponential decay or other nonlinear graph. For example, ...
- Thu Mar 01, 2018 10:31 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Salt Bridge [ENDORSED]
- Replies: 1
- Views: 299
Re: Salt Bridge [ENDORSED]
Some reactions, including concentration cells, use porous discs in the center instead of a salt bridge. This would be drawn as a single line, not a double line, in the center of the cell diagram.
- Thu Mar 01, 2018 7:57 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Fractional Reaction Orders [ENDORSED]
- Replies: 2
- Views: 412
Fractional Reaction Orders [ENDORSED]
What does it mean for there to be a fractional (for example 1.5) reaction order and how does this happen? Will we have to know/work with reactions like this on exams? Also, is having a fractional reaction order common?
- Sun Feb 25, 2018 7:23 pm
- Forum: Second Order Reactions
- Topic: Question about half-life regarding hw 15.35
- Replies: 1
- Views: 294
Re: Question about half-life regarding hw 15.35
I think you can't just do the half-life times four because the half-life is 50.5 s when [A]0=0.84 mol/L, but the half-life changes as the concentration changes, so later in the reaction it is a different value.
- Sun Feb 25, 2018 7:09 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Electrochemical Series
- Replies: 3
- Views: 585
Re: Electrochemical Series
There is one in the appendix of the textbook on page A17 if you want an example.
- Sun Feb 25, 2018 6:32 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: figuring out n and m
- Replies: 3
- Views: 546
Re: figuring out n and m
With the equations we have so far (as of end of week 7) I'm pretty sure that we need to be given the concentrations in order to calculate a numerical value for n and m.
- Sun Feb 18, 2018 10:09 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Concentration Cells
- Replies: 5
- Views: 740
Re: Concentration Cells
Concentration cells can be used to make a simple battery (using only a difference in concentration not substance) but not a very strong one
- Sun Feb 18, 2018 8:14 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Order in Cell Diagrams
- Replies: 4
- Views: 570
Re: Order in Cell Diagrams
Can you please clarify? In what context do you mean?
- Sun Feb 18, 2018 7:58 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Nernst Equation
- Replies: 4
- Views: 783
Re: Nernst Equation
The Nernst equation allows us to calculate E based on the standard electric potential, temperature, and concentrations of a redox reaction.
- Sun Feb 11, 2018 3:33 pm
- Forum: Balancing Redox Reactions
- Topic: E cell potiential
- Replies: 2
- Views: 292
Re: E cell potiential
Yes, n is referring to the number of moles of electrons transferred in the redox reaction, and this number can be found in the balanced equation.
- Sun Feb 11, 2018 3:26 pm
- Forum: Balancing Redox Reactions
- Topic: Nature
- Replies: 5
- Views: 690
Re: Nature
In nature, and in the lab, all redox reactions involve both oxidation and reduction. This is due to the conservation of electrons. They have to go somewhere!
- Sun Feb 11, 2018 3:22 pm
- Forum: Balancing Redox Reactions
- Topic: Reduction and Oxidation
- Replies: 4
- Views: 503
Re: Reduction and Oxidation
When the oxidation number increases, it is being oxidized, and if the oxidation number decreases, it is being reduced.
- Sun Feb 04, 2018 10:29 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Open, closed, or isolated test 1 question
- Replies: 10
- Views: 1655
Re: Open, closed, or isolated test 1 question
A vial filled with an organic solvent is a closed system (if the vial has a lid) because no matter can enter of leave the vial, but energy can enter it by heating or cooling it.
- Sun Feb 04, 2018 6:29 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: delta G
- Replies: 3
- Views: 418
Re: delta G
I'm not really sure what you mean by minimum temperature (minimum temperature of what?), but delta G has to be zero at phase changes (ex. at boiling point and melting point) because this is the point at which the process goes from being spontaneous (negative delta G) to not spontaneous (positive).
- Sun Feb 04, 2018 3:42 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Deriving Equations
- Replies: 2
- Views: 377
Re: Deriving Equations
I'm pretty sure Dr. Lavelle told us in lecture that we did not have to be able to mathematically derive the equations for the test but that being able to do so is helpful for understanding what the equations mean so that we can apply them correctly.
- Sun Jan 28, 2018 10:03 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Isothermal Reversible
- Replies: 3
- Views: 837
Re: Isothermal Reversible
Yes, a change in temperature makes the reaction not reversible because all reversible reactions are isothermal. However, it is also possible to have an irreversible isothermal reaction.
- Sun Jan 28, 2018 9:55 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Boltzmann equation: positional entropy or thermal entropy?
- Replies: 4
- Views: 640
Re: Boltzmann equation: positional entropy or thermal entropy?
According to the textbook the Boltzmann equation calculates "statistical entropy," which I think takes both positional and thermal entropy into account.
- Sun Jan 28, 2018 9:51 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: About entropy at T=0
- Replies: 1
- Views: 290
Re: About entropy at T=0
I think that either this equation is assuming that the substance is a perfect crystal or that it is an approximation and that the positional entropy of the substance at 0K is negligible when compared with the deltaS term.
- Sun Jan 21, 2018 4:58 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Units of Standard Enthalpies of Formation
- Replies: 2
- Views: 235
Re: Units of Standard Enthalpies of Formation
My TA writes it as kJ/mole of reaction, but said it doesn't really matter if you use kJ or kJ/mol. I think just make sure to keep track of your units in your work and use that.
- Sun Jan 21, 2018 4:50 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Standard Enthalpies of Formation
- Replies: 3
- Views: 298
Re: Standard Enthalpies of Formation
Using standard enthalpies of formation and using bond enthalpies are actually two different methods of calculating the enthalpy of the reaction. If you are given standard enthalpies of formation (SEF), do the sum of the SEF of the products minus the sum of the SEF of the reactants to get the enthalp...
- Sun Jan 21, 2018 4:32 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: state property
- Replies: 3
- Views: 427
Re: state property
Heat and work are not state functions because they depend on the path taken (like distance traveled), while enthalpy is a state function because it does not depend on what happens between the initial and final states (like elevation gain). For example, if there is a reaction in which there is no cha...
- Sun Jan 14, 2018 10:32 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Extensive vs Intensive Properties
- Replies: 3
- Views: 300
Re: Extensive vs Intensive Properties
An extensive property depends on the amount of a substance there is, while an intensive property is the same no matter how much of a specific substance there is. Heat capacity depends on the amount of a substance because it takes more heat to raise the temperature of a larger sample so it is an exte...
- Sun Jan 14, 2018 7:31 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard Reaction Enthalpy Definition
- Replies: 7
- Views: 608
Re: Standard Reaction Enthalpy Definition
Also, Dr. Lavelle said that standard reaction enthalpy is at 1 atm, but my TA said it was at 1 bar. Which is it?
- Sun Jan 14, 2018 7:24 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.57
- Replies: 1
- Views: 229
Re: 8.57
You can only use the products-reactants method if you are given the heats of formation and this problem gives heats of combustion instead. The answer book manipulates (adds, reverses, multiplies by a constant) the given combustion equations until getting the equation: C2H2 + 2H2 --> C2H6. The same o...
- Sat Dec 09, 2017 7:58 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Change in Equilibrium Constant
- Replies: 2
- Views: 420
Re: Change in Equilibrium Constant
Yes the equilibrium constant will change if the stoichiometric coefficients are halved. For example for the reaction 2A <--> 3B K1 = [B]^3 / [A]^2 which is not the same as the equilibrium constant of the reaction A <--> 3/2B K2 = [B]^3/2 / [A] because the coefficients become the exponents in the K e...
- Sat Dec 09, 2017 7:19 pm
- Forum: Significant Figures
- Topic: Logarithms
- Replies: 3
- Views: 452
Logarithms
Can someone please explain the sig fig rules for logarithms? And why do they make sense? Why can't we just use the number of sig figs in the given numbers in the question?
- Sun Dec 03, 2017 6:34 pm
- Forum: Amphoteric Compounds
- Topic: d-block elements
- Replies: 1
- Views: 379
Re: d-block elements
I found a past post that might help: "Re: particular d-block metals form amphoteric oxides? Postby Chem_Mod » Sun Aug 21, 2011 10:46 am We covered metal oxides and hydroxides (e.g., NaOH) which are basic compounds. We also covered non-metal oxides (e.g., H2SO4, H3PO4, etc.,) which are acidic co...
- Sun Dec 03, 2017 6:16 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: difference in acids
- Replies: 3
- Views: 558
Re: difference in acids
Also, the Lewis definition is more general than the Bronsted definition (not all Lewis acids are Bronsted acids, but all Bronsted acids are Lewis acids)
- Sun Nov 26, 2017 7:41 pm
- Forum: Naming
- Topic: 17.31 part c
- Replies: 2
- Views: 392
Re: 17.31 part c
[Co(NH3)4(H20)2] has an overall charge of +3 and each bromide ion has a charge of -1, so three bromide ions are needed to balance the charges in this ionic compound, hence the subscript 3.
- Sun Nov 26, 2017 7:34 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 4.11
- Replies: 1
- Views: 235
Re: 4.11
The answer book says that the "arrangement of electron pairs will be trigonal bipyramidal ... the actual structure is described as a seesaw" for this problem (page 98). When classifying the shape of a molecule we do not count lone pairs, so we get seesaw, but if we did count lone pairs as ...
Re: Hg(CH3)
I looked online and could not find dimethylmercury being referred to as dimethylmercury(0). Where did you see it called that? But you are right that it is Hg(II).
- Tue Nov 14, 2017 9:14 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Chapter 4 #25
- Replies: 1
- Views: 688
Re: Chapter 4 #25
This question is actually really misleading because while it looks like you can place the Cl atoms opposite each other and therefore cancel the dipole moments if you just use the Lewis Structure, if you look at the molecule's shape you can see that this doesn't work. CH2Cl2 has four regions of elect...
- Sun Nov 12, 2017 5:10 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Trigonal Bipyramidal
- Replies: 2
- Views: 259
Re: Trigonal Bipyramidal
The non-central atoms in a trigonal bipyramidal molecule don’t have to be the same. For example PF3Cl2, where P is the central atom and both F and Cl surround it.
- Wed Nov 08, 2017 10:48 pm
- Forum: Bond Lengths & Energies
- Topic: Homework Problem 3.87
- Replies: 5
- Views: 859
Re: Homework Problem 3.87
F is the smallest atom among F, Cl, and Br, meaning that the internuclear distance between the fluorine and carbon would be the smallest even while ignoring any effects by the relative electronegativities. This means the F-C bond is the shortest of the three.
- Sat Nov 04, 2017 10:19 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Trigonal planar vs. Trigonal bipyramidal
- Replies: 4
- Views: 1740
Re: Trigonal planar vs. Trigonal bipyramidal
Here's some example images to help you visualize the difference between trigonal planar and trigonal bipyramidal: Trigonal Planar: https://upload.wikimedia.org/wikipedia/commons/6/6c/AX3E0-3D-balls.png Trigonal Bipyramidal: https://upload.wikimedia.org/wikipedia/commons/3/3a/Trigonal-bipyramidal-3D-...
- Wed Nov 01, 2017 8:59 pm
- Forum: Ionic & Covalent Bonds
- Topic: Ionic vs. Covalent Bonds
- Replies: 3
- Views: 811
Re: Ionic vs. Covalent Bonds
Also, some molecules with polar covalent bonds are overall nonpolar due to their symmetry. For example, CO2 is a nonpolar molecule even though the C to O bond is polar covalent (electronegativities: 3.5-2.5=1 which is between .5 and 1.5). This is because it is symmetrical, so the partial negative ch...
- Sun Oct 29, 2017 9:36 pm
- Forum: Lewis Structures
- Topic: Placement [ENDORSED]
- Replies: 6
- Views: 778
Re: Placement [ENDORSED]
What if there are multiple of the atom with the lowest ionization energy (for example 4 carbon atoms)? Which one would be central and how would they be arranged? Can we predict the shape of the Lewis structures for complicated molecules?
- Tue Oct 24, 2017 10:27 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Shells, Subshells, and Orbitals
- Replies: 4
- Views: 797
Re: Shells, Subshells, and Orbitals
To elaborate on orbitals, there are three orbitals in the p-subshell (px, py, and pz) that are determined by the ml quantum number. You can think of the orbitals as the different "orientations" that the subshell can have. The d-subshell has five orbitals. Each orbital can have two electron...
- Sun Oct 22, 2017 1:29 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Cartesian axes [ENDORSED]
- Replies: 2
- Views: 1019
Re: Cartesian axes [ENDORSED]
The cartesian axes are the x-,y-, and z-axes representing the three physical dimensions. In other words they are directions in space (forward-backward, left-right, and up-down). The orientations of the orbitals are defined along these axes in order to make the math work. There is no "universal&...
- Wed Oct 18, 2017 9:45 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Ground state vs excited state
- Replies: 3
- Views: 750
Re: Ground state vs excited state
If an electron is in its ground state, for example 1s, and is excited, is there a difference in energy between being excited to the 2s vs 2p orbitals? We were told in class that the first quantum number is the one that determines energy but also that electrons fill the s orbitals before the p orbita...
- Sat Oct 14, 2017 9:17 pm
- Forum: Properties of Electrons
- Topic: Wave Properties of Electrons [ENDORSED]
- Replies: 4
- Views: 627
Re: Wave Properties of Electrons [ENDORSED]
Does that mean that electrons have different wavelengths? How would you find the wavelength or frequency of an electron?
- Tue Oct 10, 2017 9:02 pm
- Forum: Properties of Light
- Topic: Problem 1.4
- Replies: 3
- Views: 501
Re: Problem 1.4
I'm pretty sure that this is a trick question and none of the answers are correct (but I think the wording is open to the possibility of there being no correct answer) A) This isn't true because all EM radiation travels at a constant speed in a vacuum and they did not say that there was a medium B) ...
- Sat Oct 07, 2017 5:53 pm
- Forum: Significant Figures
- Topic: Sig Figs in a problem with addition & multiplication [ENDORSED]
- Replies: 5
- Views: 3147
Re: Sig Figs in a problem with addition & multiplication [ENDORSED]
If the problem has both addition and multiplication, do we use the addition/subtraction rule or the multiplication/division rule for determining the number of sig figs in the final answer?
- Thu Oct 05, 2017 5:50 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Formula Units Usage
- Replies: 3
- Views: 747
Re: Formula Units Usage
A formula unit is a group of ions. You can think of it as a "molecule" of an ionic compound such as NaCl (table salt). When the textbook asks for the amount of a compound in formula units it is asking for a count of formula units or "How many formula units are there in this sample.&qu...

