Search found 49 matches
- Sat Mar 17, 2018 9:22 pm
- Forum: General Rate Laws
- Topic: Test 3 Q3- Friday discussion
- Replies: 1
- Views: 496
Re: Test 3 Q3- Friday discussion
Yup, that's the right answer.
- Sat Mar 17, 2018 8:57 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Electron Transfer from Oxidation Number
- Replies: 1
- Views: 372
Re: Electron Transfer from Oxidation Number
From what I can gather, the only molecule in this reaction that has an overall charge is 02. Each one is -2, so overall it is -4, and because there are 6 02, there is a charge of -24 on it. Only the reactant side has a a negative charge, so I would put 24 electrons on the other side to balance out t...
- Sat Mar 17, 2018 8:48 pm
- Forum: Balancing Redox Reactions
- Topic: Notation question
- Replies: 2
- Views: 515
Re: Notation question
Apparently NaN3 is an ionic compound, so the Na and N3 are attracted by their difference in charges. Na has an oxidation state of +1, but with the N3, we have to figure out the overall charge of each nitrogen via formal charges. How they have it N=N=N, the outermost Nitrogens have a formal charge of...
- Sat Mar 17, 2018 8:19 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Catalysts
- Replies: 3
- Views: 581
Re: Catalysts
We know if there is a catalyst if it was present in the reactants in the first step, and is present as a product in the last step. It is never consumed. This is opposed to an intermediate, which is formed in the first step and later consumed in a later step. A+B->C C+D->E+B A+D->E In this, B is the ...
- Sat Mar 17, 2018 8:12 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 14.15a
- Replies: 2
- Views: 355
Re: 14.15a
I believe it is because the Ag(s) is acting as an electrode. Although they are both solids, it would be difficult to tell which is the electrode if you just put a comma as they are both solids, so they put a line to differentiate between the electrode and the "solution".
- Sat Mar 17, 2018 8:04 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Entropy change in volume or pressure
- Replies: 1
- Views: 426
Re: Entropy change in volume or pressure
For both equations, the temperature is not changing, isothermal. The volume and pressure for both equations aren't constant, due to PV=nRT. The pressure and volume are inversely proportional, so changing one changes the other.
- Wed Mar 14, 2018 2:44 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Can someone help me identify the oxidizing and reducing agents in this galvanic cell?
- Replies: 1
- Views: 451
Re: Can someone help me identify the oxidizing and reducing agents in this galvanic cell?
O_{3} is the oxidizing agent O_{2} is the reducing agent I found this out by "matching" what we were given in the appendix of reducing half-reactions given to us for the test. For O3/O2, OH- , the standard reduction potential of O3 + H20 + 2e -> O2 + 2OH matches For O3, H+/O2, the standar...
- Wed Mar 14, 2018 2:18 am
- Forum: Phase Changes & Related Calculations
- Topic: Peer Learning Prob on heat
- Replies: 1
- Views: 311
Re: Peer Learning Prob on heat
By setting both heats opposite to each other, did you mean making them both equal to each other?
If so, you'd need to also set one side as negative, because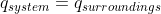
If so, you'd need to also set one side as negative, because
- Wed Mar 14, 2018 2:10 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Test 2 Question 5
- Replies: 2
- Views: 320
Re: Test 2 Question 5
Your logic is correct, Mn has the most reducing power. But the question asks to put them in order of increasing reducing power, which means you have to order them from worst reducing power to best reducing power. The way you wrote it is technically correct, with the inequality signs, Mn>Zn>Cr. If yo...
- Mon Mar 12, 2018 12:33 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Example 15.7
- Replies: 2
- Views: 304
Re: Example 15.7
I doubt it, since the method requires a graphing calculator, and we haven't gone over that method in class.
- Mon Mar 12, 2018 12:31 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Pre-equilibrium Condition
- Replies: 3
- Views: 538
Re: Pre-equilibrium Condition
It's saying that when the first step is fast, and the second step is slow, then when the first step creates the intermediate, it does this at a much faster rate than the second step can consume it. So a bottleneck is created, where you have a bunch of intermediate waiting to be consumed in the slow ...
- Mon Mar 12, 2018 12:16 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Collision Cross Section
- Replies: 2
- Views: 523
Re: Collision Cross Section
The collision cross section is important because it makes up a component of A, the pre-exponential factor. A=\sigma \bar{v} {N_{a}}^{2} The pre-exponential factor tells us the rate at which molecules collide with each other. The collision cross section plays into this because it gives us the area of...
- Sun Mar 11, 2018 11:58 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Steric requirement
- Replies: 1
- Views: 1407
Re: Steric requirement
When calculating the rate constant using the collision theory, we need to take into account the orientation of the colliding atoms/molecules. This is the steric requirement, P. This steric factor is usually always less than 1 because the steric requirement lowers the probability of the reaction occu...
- Mon Mar 05, 2018 12:51 am
- Forum: General Rate Laws
- Topic: Homework Problem 15.19C
- Replies: 5
- Views: 648
Re: Homework Problem 15.19C
I was able to get 2.85x10^(12) by converting everything from mmol into mol. By dividing everything by 1000 first, the initial reactant concentrations and initial rate, you get k=2.85x10^(12). Only problem is that the book states the answer in mmol still, so this may possibly be an error in the answe...
- Mon Mar 05, 2018 12:38 am
- Forum: First Order Reactions
- Topic: Half-life of first order reactions
- Replies: 3
- Views: 465
Re: Half-life of first order reactions
Yup, that is exactly correct!
From the book, "The half-life of a reactant that decays in a first-order reaction is characteristic of the reaction and independent of the initial concentration. It is inversely proportional to the rate constant of the reaction."
From the book, "The half-life of a reactant that decays in a first-order reaction is characteristic of the reaction and independent of the initial concentration. It is inversely proportional to the rate constant of the reaction."
- Sun Feb 18, 2018 9:37 pm
- Forum: Balancing Redox Reactions
- Topic: Basic v Acidic
- Replies: 2
- Views: 360
Re: Basic v Acidic
They will always tell us whether the reaction is basic or acidic in the question, and from that knowledge, we will know when to use H+ (acidic) or OH- (basic) to balance out the reaction. For either one, though, you'll have to use H20 to balance out the reaction.
- Sun Feb 11, 2018 10:15 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Problem 9.75 Clarification
- Replies: 1
- Views: 247
Re: Problem 9.75 Clarification
Residual Entropy is the entropy entirely dependent on the positional disorder when T=0. All entropy still available can't be due to the Temperature, but there is still an inherent entropy due to the different microstates of the substance. A trans-isomer is pretty much an isomer where 2 parts are on ...
- Sun Feb 11, 2018 9:42 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Sublimation
- Replies: 3
- Views: 521
Re: Sublimation
The only places where I've seen Enthalpy of Sublimation for a specific substance used is when we are using mean bond enthalpies to calculate the enthalpy for the reaction. We would use sublimation when we need to create a new molecule in a gaseous form, but one of the reactants is in a solid state. ...
- Sun Feb 11, 2018 9:32 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 9.43
- Replies: 1
- Views: 382
Re: 9.43
We can't use that equation because we don't know the final temperature, we are only given the initial temperatures of both bodies of water. That means we can use this equation, \Delta S=nCln\frac{T_{1}}{T_{2}} , if we know the final temperature, because then we would know the final temperatures of b...
- Mon Feb 05, 2018 12:20 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Question: Refrigerator Cooling
- Replies: 3
- Views: 539
Re: Question: Refrigerator Cooling
I think it's only because the surroundings in this case are so much more larger and have much more volume as compared to the refrigerator and what it produces. To cool the whole room down would require a large amount of energy, which would be theoretically possible if the refrigerator were large eno...
- Mon Feb 05, 2018 12:16 am
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: 9.43
- Replies: 2
- Views: 482
Re: 9.43
Because we need to know the entropy of both the system (20 C) and the surroundings (50 C), we need to use the equation for finding the entropy with the final and initial temperatures. Unfortunately, they don't give us the final Temperature, so we have to find it ourselves. So you set both of the equ...
- Mon Feb 05, 2018 12:09 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 9.19 Finding standard entropy of water
- Replies: 1
- Views: 271
Re: 9.19 Finding standard entropy of water
Because Entropy is a state function, we can take any amount of paths to get to the final value. Because we have water going from a liquid to a gas, we have to take into account the phase change, so we need to add the equations of the liquid reaching the boiling point and its vaporization. From the g...
- Sun Jan 28, 2018 10:53 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Test question [ENDORSED]
- Replies: 16
- Views: 1788
Re: Test question [ENDORSED]
I put that the First Law of Thermodynamics states that the internal energy of an isolated system will not change. I also put ) just in case. Hopefully that's specific enough.
just in case. Hopefully that's specific enough.
- Sun Jan 28, 2018 10:50 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Heat capacity [ENDORSED]
- Replies: 4
- Views: 539
Re: Heat capacity [ENDORSED]
No, any heat capacities, along with any relevant constants/equations, will be provided to us with every test on an equations sheet.
- Sun Jan 28, 2018 10:24 pm
- Forum: Phase Changes & Related Calculations
- Topic: degeneracy [ENDORSED]
- Replies: 7
- Views: 840
Re: degeneracy [ENDORSED]
Degeneracy is the total number of ways of achieving a given energy state. In the dogbone example Dr. Lavelle showed us, we could could calculate W with the info given by W_{N}=2^{N} . N is the number of particles, molecules, etc., while the 2 was the number of equivalent states. So if there was 1 pa...
- Sun Jan 21, 2018 6:18 pm
- Forum: Phase Changes & Related Calculations
- Topic: 8.39
- Replies: 1
- Views: 165
Re: 8.39
Yes, since the solid is already at its melting point, you can start with the phase change calculation, and then add that to the heat needed to get the water to the specific temperature.
- Sun Jan 21, 2018 6:13 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Cv vs. Cp [ENDORSED]
- Replies: 13
- Views: 11889
Re: Cv vs. Cp [ENDORSED]
Cp is the heat capacity for something at a constant pressure, and would be equal to \Delta Cp=\Delta H/\Delta T Cv is the heat capacity for something at a constant volume, and would be equal to \Delta Cv=\Delta U/\Delta T You'll know when to use a particular heat capacity because they'll specify in ...
- Sun Jan 21, 2018 5:54 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 8.25
- Replies: 3
- Views: 536
Re: 8.25
The regular heat equation for any system/surroundings is Qsystem=-Qsurroundings, because the amount of energy must be conserved between the 2 environments, due to the Law of the Conservation of Energy. In this case, the reaction=system, and surroundings=calorimeter, so Qreaction= -Qcalorimeter. Then...
- Sun Jan 14, 2018 10:38 pm
- Forum: Phase Changes & Related Calculations
- Topic: Heat Capacity
- Replies: 4
- Views: 556
Re: Heat Capacity
And extensive property is a property that changes based on how much there is of the sample- it is based on weight, for example, mass, volume, etc. An intensive property is a property that is an intrinsic quality of the sample- it does not change based on weight, for example, density, hardness, etc. ...
- Sun Jan 14, 2018 10:37 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Extensive vs Intensive Properties
- Replies: 3
- Views: 300
Re: Extensive vs Intensive Properties
And extensive property is a property that changes based on how much there is of the sample- it is based on weight, for example, mass, volume, etc. An intensive property is a property that is an intrinsic quality of the sample- it does not change based on the weight, for example, density, hardness, e...
- Sun Jan 14, 2018 10:24 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard State
- Replies: 2
- Views: 223
Re: Standard State
Elements are in their standard state when they are in their most stable phase at the temperature of interest, which is usually 25 Celsius, while exposed to a constant pressure of 1 atm (They must also be 1 mole if they are a solution). So you can look at a periodic table at 25 C. and check the phase...
- Sun Dec 03, 2017 10:54 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Oxidation States
- Replies: 4
- Views: 3980
Re: Oxidation States
Are you saying if you want to find the oxidation state for anything, or just the transition metal in the coordination complex? For many elements, you can find the oxidation state by imagining what would happen if the element were in an ionic bond, and were to have a full octet. How many electrons wo...
- Sun Dec 03, 2017 10:39 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Ph, Poh, and kw
- Replies: 2
- Views: 3882
Re: Ph, Poh, and kw
The Kw constant is another name for the autoprotolysis constant. Autoprotolysis is a reaction where water naturally decomposes into hydronium and hydroxide, due to the amphiprotic nature of water allowing proton transfer between the same type of molecule. The equilibrium constant would be found like...
- Sun Dec 03, 2017 10:15 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 11.47
- Replies: 1
- Views: 357
Re: 11.47
Because we are given grams of the reactant, we have to convert to concentration by converting the grams to moles, and then dividing that by the vessel volume. 1.0g/208.22 moles --> .00480 mole/.250 Liters ---> .0192 mol./L. Then we would insert this concentration (.0192) into the ICE box, in the &qu...
- Sun Dec 03, 2017 9:56 pm
- Forum: Hybridization
- Topic: Pi-Bonds and their interactions
- Replies: 2
- Views: 400
Re: Pi-Bonds and their interactions
A sigma bond has overlapping orbitals in a straight line, while pi bonds have orbitals that overlap when the p orbitals are parallel to each other. When you have a double bond, it's a sigma bond, along with the other p orbitals oriented so that they can also create a bond. The other post explained i...
- Sun Nov 26, 2017 5:08 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium Constant units
- Replies: 2
- Views: 323
Re: Equilibrium Constant units
K is a ratio between the concentrations/partial pressures of the reactants and products when they have reached equilibrium. The units of the products and reactants would cancel each other out. Also, K being a ratio would mean its just a numerical relationship between two different concentration/part...
- Sun Nov 26, 2017 4:55 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Some clarification about ligands
- Replies: 5
- Views: 639
Re: Some clarification about ligands
Ligand is another name for a Lewis base which bonds to a central metal atom/ion. A Lewis base is able to do this by the presence of lone pairs, allowing the atom to "donate" its electrons and covalently bond to the central metal atom. This bond is called a coordination covalent bond, and t...
- Sun Nov 19, 2017 11:58 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: seesaw shape
- Replies: 1
- Views: 478
Re: seesaw shape
The see-saw shape is based off of the trigonal bipyramidal, as the molecule in question has 5 areas of electron density. But then you would just need to replace one of the bonds with a lone pair, and in this case it would be the equatorial bond, as you would only be strongly repelling against 2 axia...
- Sun Nov 19, 2017 10:18 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Angular
- Replies: 2
- Views: 233
Re: Angular
Yes, they're the same thing.
And for 4.5 a, it would be angular (bent) since the central Cl atom has a double bond with each O atom along with a lone pair. This lone pair repels the other bonds as far away as possible to attain the lowest energy, resulting in the bent shape similar to H20.
And for 4.5 a, it would be angular (bent) since the central Cl atom has a double bond with each O atom along with a lone pair. This lone pair repels the other bonds as far away as possible to attain the lowest energy, resulting in the bent shape similar to H20.
- Sun Nov 05, 2017 7:57 pm
- Forum: Lewis Structures
- Topic: HW 3.59 PART C
- Replies: 1
- Views: 182
Re: HW 3.59 PART C
If you drew N with double bonds to both O, then you would have 10 electrons for N, since you also have to draw a single bond from N to the other O that has Cl attached. You can only have one double bond with 1 O, because then you would be able to have 4 bonds connecting to N, fulfilling the octet ru...
- Sun Nov 05, 2017 7:48 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: 3.49
- Replies: 3
- Views: 646
Re: 3.49
When solving for formal charge, the L is the # of electrons in the bond pair, so it would be 2; you don't count the whole pair as 1.
So if we do that, then O would be 6 -(2+ 6/2)= +1, and N would be 5 -(2 + 6/2)= 0
So if we do that, then O would be 6 -(2+ 6/2)= +1, and N would be 5 -(2 + 6/2)= 0
- Sun Oct 29, 2017 11:36 pm
- Forum: Lewis Structures
- Topic: Lewis Structure and Resonance
- Replies: 1
- Views: 284
Re: Lewis Structure and Resonance
While resonance does have to do with the possibility of multiple bonds, it is important to match up the correct number of e- with each resonance bond. If you are talking about a resonance hybrid, when you combine all the resonance structures, you still have to include the matching number of lone pai...
- Sun Oct 29, 2017 11:04 pm
- Forum: Resonance Structures
- Topic: Resonance Structures [ENDORSED]
- Replies: 3
- Views: 556
Re: Resonance Structures [ENDORSED]
A resonance structure would just be the Lewis structure of all the possible configurations of the bonds in a molecule. This is signified by a double arrow between each possible Lewis structure, showing the possible resonance structures of the molecule. But the best representation of a molecule would...
- Sun Oct 22, 2017 6:07 pm
- Forum: DeBroglie Equation
- Topic: Exercise 1.42 [ENDORSED]
- Replies: 2
- Views: 488
Re: Exercise 1.42 [ENDORSED]
I would assume you need to use the de Broglie Equation, \lambda =h/m_{h}*v They give us the velocity, and we already know what constant h is, so we would just need to know the mass of a helium atom. We would get that by dividing the molar mass of Helium, 4.0026, by Avogadro's constant, 6.02214*10^{2...
- Tue Oct 17, 2017 6:18 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Question 1.15
- Replies: 6
- Views: 495
Re: Question 1.15
v=R(1/n_{1}^{2}-1/n_{2}^{2}) is just another way of writing \Delta E= E_{final}- E_{initial} When we convert the \Delta E equation, the E_{final} corresponds to n_{1} , and E_{initial} corresponds to n_{2} We know that n_{1}=1 , the final energy level, and n_{2}=3 , the initial energy level...
- Sun Oct 15, 2017 5:07 pm
- Forum: Properties of Electrons
- Topic: Atomic Spectra and Energy Levels
- Replies: 3
- Views: 497
Re: Atomic Spectra and Energy Levels
The equation for the energy of an electron at any energy level is 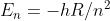
From this equation, we know that as n goes higher, the energy will keep getting smaller. When n is equal to infinity, the Energy will be equal to 0, since dividing by infinity results in 0.
From this equation, we know that as n goes higher, the energy will keep getting smaller. When n is equal to infinity, the Energy will be equal to 0, since dividing by infinity results in 0.
- Sun Oct 15, 2017 4:44 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Variation of Empirical Equation of H-atom [ENDORSED]
- Replies: 2
- Views: 537
Re: Variation of Empirical Equation of H-atom [ENDORSED]
\Delta E = E_{final}-E_{initial} so we can plug in E_{n}=-hR/n^2 for the final and initial eqation, \Delta E= (-hR/n_{1}^2) - (-hR/n_{2}^2) We can make E_{f} have n_{1} because it is the final and lower energy level, which is the case when the electron is moving down an energy level...
- Sun Oct 08, 2017 10:55 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3640201
Re: Post All Chemistry Jokes Here
All these jokes were noble attempts, yet I didn't really react to any of them.
- Sun Oct 08, 2017 10:25 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3640201
Re: Post All Chemistry Jokes Here
What's a graduate student's favorite element?
Alumni-um
Alumni-um

