Search found 50 matches
- Sat Mar 17, 2018 4:44 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: UA Practice Final 1D and 1E
- Replies: 3
- Views: 506
Re: UA Practice Final 1D and 1E
Because the anode metal is a solid, the Ecell won't be affected. Ecell is dependent on concentration and temperature.
- Sat Mar 17, 2018 4:28 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Finding E
- Replies: 3
- Views: 584
Re: Finding E
If you flip the sign, use Ecell=Eanode+Ecathode. If you don't flip the sign, use Ecell= Ecathode-Eanode.
- Sat Mar 17, 2018 3:58 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 9.13
- Replies: 2
- Views: 489
Re: 9.13
Both equations are also given on the constants sheet. And the C value can be Cv at constant volume, or Cp at constant pressure.
- Sat Mar 10, 2018 11:30 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Finding n
- Replies: 15
- Views: 1886
Re: Finding n
If a half reaction is multiplied by 2 to get an equal number of moles of electrons in both half reactions to be able to cancel each other out, then yes n=2.
- Sat Mar 10, 2018 11:16 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.53
- Replies: 3
- Views: 750
Re: 8.53
How do you know to use the equation nR\Delta T) ?
?
- Sat Mar 10, 2018 10:57 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Test 2 Question 2
- Replies: 4
- Views: 617
Re: Test 2 Question 2
Adding on to the comment above, you can also use the equation 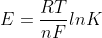 to solve for K.
to solve for K.
- Sun Mar 04, 2018 4:09 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3645801
Re: Post All Chemistry Jokes Here
Why can't chemists prank their friends?
They lack the element of surprise.
They lack the element of surprise.
- Sun Mar 04, 2018 3:35 pm
- Forum: Second Order Reactions
- Topic: Slope of second order reaction
- Replies: 3
- Views: 654
Re: Slope of second order reaction
The integrated rate laws of the zero and first-order have a -kt, while the second-order has a +kt. Since the integrated rate laws correspond to y=mx+c (m=k), the slope depends on k.
- Sun Mar 04, 2018 2:39 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Straight line
- Replies: 7
- Views: 1767
Re: Straight line
Adding on to the comment above, -k would be for zero and first-order reactions, and k would correspond to second-order reactions.
- Sat Feb 24, 2018 10:00 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: What other elements could be used besides Pt?
- Replies: 5
- Views: 719
Re: What other elements could be used besides Pt?
How do you know when to use Pt or graphite?
- Sat Feb 24, 2018 9:46 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Strongly Reducing Metals
- Replies: 5
- Views: 688
Re: Strongly Reducing Metals
The opposite goes for oxidizing agents; the more positive the standard cell potential, the stronger the oxidizing agent.
- Sat Feb 24, 2018 9:38 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Creating a Cell Diagram with solids
- Replies: 2
- Views: 385
Re: Creating a Cell Diagram with solids
Are there any other special cases like liquid Hg where it can be considered as an electrode?
- Sun Feb 18, 2018 11:46 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Salt Bridge
- Replies: 4
- Views: 439
Re: Salt Bridge
A salt bridge allows the flow of ions to complete the electrical circuit without affecting the cell potential.
- Sun Feb 18, 2018 11:27 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: electrolytic cells
- Replies: 3
- Views: 677
Re: electrolytic cells
Electrolysis occurs in electrochemical cells and drives reactions in their nonspontaneous directions through an electric current.Electrolysis can be used to to predict how much products form from an electric current flow.
- Sun Feb 18, 2018 10:59 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Nernst Equation
- Replies: 6
- Views: 949
Re: Nernst Equation
If you were confused with the subscript, page 571 says, "The subscript r is always the signal that we are using this 'molar' form."
- Thu Feb 08, 2018 11:12 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 11.17
- Replies: 10
- Views: 1079
Re: 11.17
Here is the link to the errors in the solution manual for future reference!
https://lavelle.chem.ucla.edu/wp-conten ... rs_6Ed.pdf
https://lavelle.chem.ucla.edu/wp-conten ... rs_6Ed.pdf
- Thu Feb 08, 2018 11:07 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Spontaneity
- Replies: 9
- Views: 1287
Re: Spontaneity
On page 348 of the textbook, there is a helpful diagram that shows that a negative change in Gibbs free energy is spontaneous.
- Thu Feb 08, 2018 10:53 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: exothermic
- Replies: 5
- Views: 745
Re: exothermic
Since the flask is getting colder, it is an endothermic reaction because the flask(as the surroundings) is losing heat. Therefore, the heat from the flask is going into the reaction.
- Sun Feb 04, 2018 3:39 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: How do we find out if a system is favorable? [ENDORSED]
- Replies: 7
- Views: 4999
Re: How do we find out if a system is favorable? [ENDORSED]
Adding to the comment above, the system is spontaneous when delta G is negative because G is energy, and decreases in energy are spontaneous.
- Sun Feb 04, 2018 3:34 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 9.65
- Replies: 6
- Views: 767
Re: 9.65
The compound becomes more unstable when the change in entropy is negative and temperature increases. So, after the standard entropies of formation are calculated, PCl5 would be the compound that is less stable since it is negative.
- Sun Feb 04, 2018 3:22 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Reversible systems
- Replies: 7
- Views: 914
Re: Reversible systems
Yes, you would calculate the change in entropy for the change in volume and the change in pressure, and add the entropy changes together.
- Sun Jan 28, 2018 2:29 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Adiabatic system
- Replies: 6
- Views: 768
Re: Adiabatic system
Adding on to the post above, pg. 268 of the textbook says that in an adiabatic system, the change in internal energy is equal to the work done.
- Sun Jan 28, 2018 2:25 pm
- Forum: Calculating Work of Expansion
- Topic: Homework 8.11
- Replies: 6
- Views: 734
Re: Homework 8.11
Keep in mind that for reversible expansion processes, the work done is the maximum work possible, therefore more work is done compared to irreversible expansion processes.
- Sun Jan 28, 2018 1:46 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3645801
Re: Post All Chemistry Jokes Here
What do hungry chemists eat?
Gram (Graham) crackers
Gram (Graham) crackers
- Sun Jan 21, 2018 10:56 am
- Forum: Calculating Work of Expansion
- Topic: Work done on / work done by system
- Replies: 5
- Views: 596
Re: Work done on / work done by system
When work is done to the system, energy is added and work is positive. When work is done by the system, energy leaves the system and work is negative.
- Thu Jan 18, 2018 10:46 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: open system
- Replies: 6
- Views: 729
Re: open system
Another example is an uncovered pot of hot water. Matter is leaving the system through the steam, and energy as heat is also lost.
- Thu Jan 18, 2018 10:33 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Reversible, Irreversible
- Replies: 4
- Views: 469
Re: Reversible, Irreversible
Also, keep in mind that a reversible process does more work than an irreversible process.
- Sun Jan 14, 2018 11:23 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Question about Question 8.7
- Replies: 3
- Views: 531
Re: Question about Question 8.7
On the other hand, if work was done by the system, the internal energy of the system would have decreased.
- Sun Jan 14, 2018 10:50 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Systems
- Replies: 7
- Views: 935
Re: Systems
An example would be a hot cup of water. Heat from the hot water is released into its surroundings, and matter can be exchanged by drinking some of the water, or through the steam that is released into the air.
- Sun Jan 14, 2018 10:38 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Closed system and Midterm
- Replies: 3
- Views: 889
Re: Closed system and Midterm
In section 8.1 of the book, it gives an example of a closed system: a cold pack. Energy can be exchanged, but not matter.
- Sat Dec 09, 2017 2:01 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Chemical Equilibrium Part 2 Module Question #27
- Replies: 2
- Views: 473
Re: Chemical Equilibrium Part 2 Module Question #27
Adding on, you should get 0.05 M H2O for the initial concentration, and you know x=0.040 from H2. The equilibrium concentration for H2O is 0.05-x, and plugging in x you would get 0.01 M H2O.
- Sat Dec 09, 2017 1:01 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Ligand Order
- Replies: 2
- Views: 344
Re: Ligand Order
Yes, ligands are written in alphabetical order when writing the formula. Toolbox 17.1 goes into detail about writing chemical formulas.
- Sat Dec 02, 2017 11:48 pm
- Forum: Amphoteric Compounds
- Topic: Amphoteric and amphiprotic compounds
- Replies: 4
- Views: 634
Re: Amphoteric and amphiprotic compounds
An amphiprotic example would be water since there are hydrogen atoms and is able to donate a proton, and the oxygen has lone pairs and is able to accept a proton.
- Sat Dec 02, 2017 11:27 pm
- Forum: Amphoteric Compounds
- Topic: amphoteric properties
- Replies: 3
- Views: 359
Re: amphoteric properties
Substances that are amphoteric can act as an acid or base, so they can donate protons or accept protons.
- Sun Nov 26, 2017 11:38 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Determining Molecular Shape
- Replies: 3
- Views: 288
Re: Determining Molecular Shape
"Because single bonds and multiple bonds are treated as equivalent in the VSEPR model, it does not matter which of the Lewis structures contributing to a resonance structure we consider" (pg. 112).
- Sun Nov 26, 2017 11:32 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lone Pairs vs. Bonding Pairs
- Replies: 6
- Views: 922
Re: Lone Pairs vs. Bonding Pairs
Bonding pairs are held in place by two atoms unlike lone pairs, so lone pairs are able to take up a larger space.
- Sun Nov 19, 2017 1:53 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bent or linear?
- Replies: 11
- Views: 2022
Re: Bent or linear?
Adding on, it would be linear because single and multiple bonds are treated as the same in the VSEPR model.
- Thu Nov 16, 2017 5:32 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Exercise 4.7 and 4.9
- Replies: 2
- Views: 252
Re: Exercise 4.7 and 4.9
Since lone pairs have a greater electron repulsion than electrons in bonds, these lone pairs push the bonded atoms closer together.
- Sun Nov 12, 2017 3:48 pm
- Forum: Bond Lengths & Energies
- Topic: Bond Length
- Replies: 8
- Views: 3388
Re: Bond Length
The larger the bonding atoms are, the distance between them is also larger, and the bond becomes weaker.
- Sun Nov 12, 2017 3:05 pm
- Forum: DeBroglie Equation
- Topic: When to use DeBroglie Equation
- Replies: 4
- Views: 500
Re: When to use DeBroglie Equation
This equation works for particles with momentum, and it has wave-like properties with wavelength.
- Sun Nov 05, 2017 10:08 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal Charge
- Replies: 8
- Views: 1118
Re: Formal Charge
Keep in mind that the formal charge of the model should add up to the overall charge of the atom.
- Sun Nov 05, 2017 1:39 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: d and s blocks
- Replies: 16
- Views: 2211
Re: d and s blocks
The electronic configuration is written based on the principle quantum number(n), in the order of increasing energy. S blocks have a greater n value than d blocks.
- Sun Oct 29, 2017 4:47 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Quick Question about SO4
- Replies: 8
- Views: 1333
Re: Quick Question about SO4
It can be determined by calculating the formal charge of the element. The lower the number of formal charges, the more stable the structure.
- Sun Oct 29, 2017 3:33 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Difference between subshell and orbital [ENDORSED]
- Replies: 9
- Views: 2660
Re: Difference between subshell and orbital [ENDORSED]
A wave function and orbital have the same meaning. In the textbook it says, "wavefunctions of electrons in atoms are called atomic orbitals."
- Sun Oct 22, 2017 7:15 pm
- Forum: *Shrodinger Equation
- Topic: What is a node?
- Replies: 5
- Views: 732
Re: What is a node?
Just to reiterate, keep in mind that the node is where the wavefunction passes through zero, it doesn't just reach zero.
- Sun Oct 22, 2017 6:52 pm
- Forum: Properties of Light
- Topic: Planck's Constant
- Replies: 10
- Views: 1278
Re: Planck's Constant
You also use Planck's constant in the equation relating the kinetic energy of an ejected e- to the energy of an incident photon.
- Sun Oct 15, 2017 8:01 pm
- Forum: Properties of Light
- Topic: Excitation of electrons
- Replies: 5
- Views: 573
Re: Excitation of electrons
Adding on, one photon interacts with one electron, and each photon must have enough energy to eject one electron to be absorbed. So if 1 million photons are absorbed, 1 million electrons are excited to a higher energy level.
- Sun Oct 15, 2017 7:21 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Balmer, Lyman, Paschen, and Bracket Series
- Replies: 3
- Views: 534
Re: Balmer, Lyman, Paschen, and Bracket Series
I'm still a bit confused about the Balmer and Lyman series. Are we just supposed to know that Balmer series are in the visible region and Lyman series are in the UV region?
- Wed Oct 04, 2017 11:58 pm
- Forum: Empirical & Molecular Formulas
- Topic: Trouble in finding the Molecular Formula
- Replies: 6
- Views: 2373
Re: Trouble in finding the Molecular Formula
I understand that we have to multiply the mole ratios to get as close to whole numbers as possible, but to what extent would the decimal be considered too far from a whole number? For example if I get 1.86, would I still be able to round that to 2?
- Wed Oct 04, 2017 11:12 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: E 29 part c
- Replies: 15
- Views: 3301
Re: E 29 part c
I'm confused with part d. How would I go about to find what fraction of the total mass of the sample was due to oxygen?

