Search found 50 matches
- Tue Mar 13, 2018 4:50 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: 9.31? Determining which one has a higher entropy?
- Replies: 1
- Views: 1133
Re: 9.31? Determining which one has a higher entropy?
Iodine vapor has a larger entropy than bromine vapor because the element iodine is larger and more electrons, therefore, there is more possibility of obtaining higher states.
- Tue Mar 13, 2018 4:39 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Catalyst
- Replies: 3
- Views: 454
Re: Catalyst
there would be no difference if the catalyst was homogeneous or heterogeneous that would change how the catalyst affects the activation energy or delta G.
- Tue Mar 13, 2018 4:29 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Organic Chem on Final
- Replies: 9
- Views: 1439
Re: Organic Chem on Final
There would be a high possibility that the ochem discussed in class would appear in the test because for the chem14a final with Lavelle, he had a question on hemoglobin that he briefly touched upon in lecture.
- Fri Mar 09, 2018 11:44 am
- Forum: First Order Reactions
- Topic: Help on 15.3
- Replies: 7
- Views: 1081
Re: Help on 15.3
The answer should be in mmol/Lxs and there's no need to change L. If anything, you should just convert mmol to mol to get the answer in mol/LxS.
- Fri Mar 09, 2018 10:49 am
- Forum: Zero Order Reactions
- Topic: Definition of Reaction Rate
- Replies: 4
- Views: 750
Re: Definition of Reaction Rate
The reaction rate is given in M/s because its the change in concentration over time where M is molarity which could also be written as mol/L.
- Thu Mar 01, 2018 10:35 am
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Hw 15.1 [ENDORSED]
- Replies: 7
- Views: 1108
Re: Hw 15.1 [ENDORSED]
yes, it depends on the mole to mole ratio in the reaction.
- Thu Mar 01, 2018 10:33 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell potential
- Replies: 5
- Views: 654
Re: Cell potential
The best way to determine if something is an anode or cathode, you would look if the electrons are gained or lost.
- Wed Feb 28, 2018 10:28 am
- Forum: Balancing Redox Reactions
- Topic: Oxidation state
- Replies: 4
- Views: 567
Re: Oxidation state
Yes, you have to multiply the coefficient with the charges because the charge is only for one atom/molecule so if you have multiple of the same molecules/atom then you have to multiply the charge by the coefficient.
- Wed Feb 28, 2018 10:24 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3591703
Re: Post All Chemistry Jokes Here
Did you know that you can cool yourself to -273.15˚C and still be 0K?
- Wed Feb 21, 2018 4:47 am
- Forum: Balancing Redox Reactions
- Topic: 14.5a
- Replies: 4
- Views: 545
Re: 14.5a
O 3 is being reduced because you have to balance the half reaction of O 3 -> O 2 which would have H 2 O on the O 2 side which would then force there to be two hydrogen ions on the ozone side which means that it would also have 2e- on that side too. Therefore, ozone is reduced since it is gaining ele...
- Wed Feb 21, 2018 4:41 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: #14.23 finding n [ENDORSED]
- Replies: 3
- Views: 536
Re: #14.23 finding n [ENDORSED]
In this case, n is the number of electrons involved in the reaction. You can determine it by balancing the half reactions.
- Wed Feb 14, 2018 5:45 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: stability and +/- delta G
- Replies: 1
- Views: 5643
Re: stability and +/- delta G
When the standard Gibbs free energy of formation is considered thermodynamically stable when it is negative because the pure compound is then considered to have a lower Gibbs free energy than the Gibbs free energy of the pure elements which would mean that the pure element would have the tendency to...
- Wed Feb 14, 2018 5:30 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: q==q
- Replies: 2
- Views: 437
Re: q==q
it would all depend on which side is losing heat or not. the side that loses heat would be the one with the negative sign.
- Wed Feb 14, 2018 5:27 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: 9.15 Question
- Replies: 2
- Views: 454
Re: 9.15 Question
Yes, it is because you are going from liquid to ice which is the opposite of fusion. You could also think of this in terms of the molecules gaining or losing heat since the molecule is going from liquid to ice, it is losing heat so deltaH would be negative.
- Wed Feb 14, 2018 5:24 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: 9.37d
- Replies: 1
- Views: 3081
Re: 9.37d
There is no relationship between the number of moles and entropy for 9.37d. Instead, it is because there is a tendency for disorder based on the second law of thermodynamics, therefore, the reaction would tend towards becoming more disordered. The key word in the problem is that it says it is decomp...
- Wed Feb 14, 2018 5:15 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Calculating Degeneracy
- Replies: 3
- Views: 646
Re: Calculating Degeneracy
You would calculate the degeneracy by the general formula: ^{number of molecules})
However, this is only true when you are given the number of molecules; if you are given the moles then you just do basic stoichiometry where 1 mole = 6.023x10^23 molecules.
However, this is only true when you are given the number of molecules; if you are given the moles then you just do basic stoichiometry where 1 mole = 6.023x10^23 molecules.
- Wed Feb 14, 2018 5:10 am
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Laws of Thermodynamics
- Replies: 2
- Views: 574
Re: Laws of Thermodynamics
First Law: The conservation of energy (deltaU = w + q) Second Law: nature of entropy increases over time; tend toward disorder (deltaS = q/t) Third Law: the entropy of perfect crystal at absolute zero is equal to 0 (eqn derived from second law) It is perfectly fine to think it the way you have howev...
- Wed Feb 14, 2018 5:01 am
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Relationship between Internal Energy, Temperature, Work, and Heat
- Replies: 1
- Views: 456
Re: Relationship between Internal Energy, Temperature, Work, and Heat
Isothermal processes are processes at a constant temperature throughout the whole process so the temperature remains the same at the end. This would mean that the change in temperature would be the same values subtracting from each other which would equate to 0. Furthermore, the kinetic energy and p...
- Wed Jan 31, 2018 4:32 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy [ENDORSED]
- Replies: 6
- Views: 1345
Re: Gibbs Free Energy [ENDORSED]
Gibbs free energy determines the favorability of a reaction whereas S shows the change in entropy/"disorder".
- Wed Jan 31, 2018 4:26 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: sign of entropy
- Replies: 11
- Views: 1682
Re: sign of entropy
You determine the sign of entropy by the direction of heat flow.
- Wed Jan 31, 2018 4:24 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy: State Function? [ENDORSED]
- Replies: 4
- Views: 2840
Re: Gibbs Free Energy: State Function? [ENDORSED]
Gibbs free energy is considered a state function because Gibbs free energy is defined by the equation: G= H-TS where entropy and enthalpy are considered state functions therefore Gibb free energy is a state function.
- Mon Jan 29, 2018 9:08 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs free energy equations
- Replies: 4
- Views: 631
Re: Gibbs free energy equations
They are essentially the same thing. The only difference is what element/molecule is part of the reaction.
- Mon Jan 29, 2018 8:45 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: calculating specific heat
- Replies: 5
- Views: 825
Re: calculating specific heat
Volume would not be necessary because specific heat capacity is in the units of Joules per grams and Celsius or Kelvin.
- Mon Jan 29, 2018 8:40 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: formula for standard entropy
- Replies: 5
- Views: 788
Re: formula for standard entropy
Yes, they are the same because they are both state functions.
- Mon Jan 22, 2018 7:53 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Is there a way specific way to approach into seeing if open closed or isolated?
- Replies: 9
- Views: 883
Re: Is there a way specific way to approach into seeing if open closed or isolated?
you should always consider if first energy is being transferred such as heat/work then it narrows down the possible systems to the open or closed system. after you have to consider if matter is being transferred from the system to the surrounding.
- Mon Jan 22, 2018 7:51 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: System Types
- Replies: 7
- Views: 872
Re: System Types
open system = matter and energy exchanged with the surrounding
closed system = only energy exchanged with the surrounding
isolated system = no matter or energy is exchanged with the surrounding
closed system = only energy exchanged with the surrounding
isolated system = no matter or energy is exchanged with the surrounding
- Wed Jan 17, 2018 4:44 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Hess Law
- Replies: 4
- Views: 503
Re: Hess Law
Yes, you can because enthalpy is a state function.
- Wed Jan 17, 2018 4:43 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: K vs C
- Replies: 6
- Views: 566
Re: K vs C
When you're calculating for the change in temperature, it doesn't matter what units of temperature it is in.
- Wed Jan 17, 2018 4:41 pm
- Forum: Phase Changes & Related Calculations
- Topic: 8.5
- Replies: 5
- Views: 488
Re: 8.5
Yes, it would be 524 + 340 because internal energy is the total store of energy in a system.
- Wed Jan 17, 2018 4:34 pm
- Forum: Phase Changes & Related Calculations
- Topic: 8.17
- Replies: 3
- Views: 1144
Re: 8.17
For melting, work is negative because work is done by the system. For condensation, work is positive because work is done on the system. Furthermore, enthalpy and heat are correlated with work.
- Tue Dec 05, 2017 4:42 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Difference between pH and pKa?
- Replies: 8
- Views: 1762
Re: Difference between pH and pKa?
pH is the measure of the concentration of hydrogen ions whereas pKa the acid dissociation constant and is able to predict what a molecule would do at specific pH.
- Tue Dec 05, 2017 4:21 pm
- Forum: Bronsted Acids & Bases
- Topic: Bronsted vs. Lewis
- Replies: 4
- Views: 597
Re: Bronsted vs. Lewis
For a bronsted-lowry acid, the acid is a electron donor, whereas in a lewis acid, it is an electron acceptor.
- Fri Dec 01, 2017 11:29 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Dentates
- Replies: 6
- Views: 920
Re: Dentates
You can figure out if a molecule is monodentate, bidentate, tridentate, etc if you just draw out the lewis dot structure and see where there are lone pairs on the central atom.
Re: Oxidation
You would say Iron (II) however, you only use Ferrate (II) when you are naming the coordination compound.
- Thu Nov 23, 2017 11:47 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Using the ICE Box
- Replies: 2
- Views: 356
Re: Using the ICE Box
[] indicates the concentration of something. For example, [x] is the concentration of x in molarity.
- Thu Nov 23, 2017 11:40 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Expanded octet
- Replies: 6
- Views: 695
Re: Expanded octet
You know an element has an expanded octet when it has any d orbitals. This is because d orbitals are able to hold more electrons than s and p orbitals.
- Thu Nov 16, 2017 12:32 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angle
- Replies: 7
- Views: 979
Re: Bond Angle
Usually, you would just memorize the bond angles for the standard molecular shapes and when there is a lone pair, you know that the angles would be slightly less than the standard bond angle.
- Thu Nov 16, 2017 12:26 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 4.1 Lone pairs
- Replies: 6
- Views: 875
Re: 4.1 Lone pairs
In the second diagram posted above, can someone please explain the significance of the thicker line and the dotted line? The thicker line indicates that the bond is towards us and the dotted line shows that the bond is going away from us. This is to help represent the 3D shape of the molecule which...
- Fri Nov 10, 2017 10:14 pm
- Forum: Lewis Structures
- Topic: Shape
- Replies: 5
- Views: 589
Re: Shape
As long as you have the correct electron distribution and number of electrons, the shape shouldn't matter that much to Dr. Lavelle.
- Fri Nov 10, 2017 2:57 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polar vs nonpolar vs ionic
- Replies: 11
- Views: 28818
Re: Polar vs nonpolar vs ionic
yes, it's covalent because the electronegativity difference is literally 0, where its considered nonpolar.
- Mon Nov 06, 2017 9:40 pm
- Forum: Octet Exceptions
- Topic: Noble gas exception
- Replies: 4
- Views: 824
Re: Noble gas exception
Unlike s and p orbitals, d orbitals can expand beyond the octet rule and xenon is so low on the periodic table, therefore, Xe is able to hold more than 8 electrons.
- Mon Nov 06, 2017 9:34 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Midterm
- Replies: 8
- Views: 927
Re: Midterm
No, only fundamentals and chapter 1-3 are going to be on the midterm.
- Sun Oct 22, 2017 4:04 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: 4s to 3d
- Replies: 5
- Views: 698
Re: 4s to 3d
The reason why the two statements contradict is due to the fact that 4s orbital is lower in energy when 3d is only partially filled however when 3d is completely filled then it becomes lower than the 4s orbital.
- Sat Oct 21, 2017 9:22 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Periodic Table
- Replies: 2
- Views: 300
Re: Periodic Table
This is because for 3d and 4s orbitals, 4s orbitals must all be filled for 3d to be completely filled too.
- Sat Oct 21, 2017 5:03 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Electric Spin
- Replies: 5
- Views: 526
Re: Electric Spin
When you draw the electron configuration, it will determine if the electron is spin up or down.
- Sat Oct 21, 2017 4:52 pm
- Forum: DeBroglie Equation
- Topic: Should velocity always be in m.s. when solving? [ENDORSED]
- Replies: 11
- Views: 2001
Re: Should velocity always be in m.s. when solving? [ENDORSED]
Yes, velocity should be in meters per second.
- Mon Oct 16, 2017 4:21 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Homework Problem 1.15
- Replies: 4
- Views: 573
Re: Homework Problem 1.15
After you solve for frequency, you use Rydberg's formula which is v (frequency) = R( -
-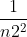 ). You always assume that n1 = 1, so you just plug in Ryberg's constant, n1 and frequency to solve for n2.
). You always assume that n1 = 1, so you just plug in Ryberg's constant, n1 and frequency to solve for n2.
- Sat Oct 14, 2017 3:34 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Change in energy of an electron [ENDORSED]
- Replies: 3
- Views: 436
Re: Change in energy of an electron [ENDORSED]
When we're solving for the change in energy, it gives us the energy emitted by the electrons because of E (photon) - E (energy remove e-) = 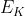 which is the energy of the electron emitted.
which is the energy of the electron emitted.
- Sat Oct 14, 2017 3:15 pm
- Forum: Photoelectric Effect
- Topic: Conversions for units in work function [ENDORSED]
- Replies: 2
- Views: 432
Re: Conversions for units in work function [ENDORSED]
Lavelle said in class that it would be given to us with the constant but my TA said that we should still know the conversion factors for future reference.
- Wed Oct 11, 2017 4:41 pm
- Forum: Properties of Light
- Topic: Clarification on C=λv
- Replies: 10
- Views: 4308
Re: Clarification on C=λv
Wavelength and frequency are inversely proportional to each other which means if frequency increases then wavelength decreases or vice versa.

