Search found 53 matches
- Sat Mar 17, 2018 10:25 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Thermodynamically stable vs Kinetically stable
- Replies: 1
- Views: 594
Re: Thermodynamically stable vs Kinetically stable
https://www.dropbox.com/s/l2i660irqroxn6k/IMG_6189.JPG?dl=0 You draw a graph like this. P1 refers to product 1, P2 refers to product 2. A is the reactant. The two high peaks are two transition states. You can see that P1 is thermodynamically more stable than P2, so P1 is more favorable in thermodyn...
- Sat Mar 17, 2018 10:17 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 14.97
- Replies: 1
- Views: 317
Re: 14.97
Ka is the acidic constant, which means it is for acids dissociation reaction. in acid dissociation reaction, H+ is dissociated. Therefore, H+ will always be on the product side. In this way, you write down the whole reaction happens. For example, in practice exam, HF\Leftrightarrow H^{+}+F^{-} . Ka ...
- Sat Mar 17, 2018 10:10 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.73a - Resonance of Benzene
- Replies: 2
- Views: 436
Re: 8.73a - Resonance of Benzene
I think if we are given the resonance bond enthalpy for benzene, we should use that value since it is the real true value for benzene.
- Sat Mar 17, 2018 10:06 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: When do you use LNQ vs LogQ?
- Replies: 1
- Views: 820
Re: When do you use LNQ vs LogQ?
When you use equation  , you use lnQ.
, you use lnQ.
When you use equation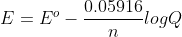 , which is the simpler equation for condition under 298K, you use logQ.
, which is the simpler equation for condition under 298K, you use logQ.
When you use equation
- Sun Mar 11, 2018 9:42 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: 15.53 (c)
- Replies: 1
- Views: 314
Re: 15.53 (c)
The second step is the slow step, so rate is determined by this step: rate = k[NO3][CO].
And NO3 is formed from step 1, so the rate = k[NO2]^2[CO].
CO cannot be eliminated from the expression since it appears in the slow step.
I do not get why you [NO3] = k1[NO2]^2 / k2[CO].
And NO3 is formed from step 1, so the rate = k[NO2]^2[CO].
CO cannot be eliminated from the expression since it appears in the slow step.
I do not get why you [NO3] = k1[NO2]^2 / k2[CO].
- Sun Mar 11, 2018 9:28 pm
- Forum: General Rate Laws
- Topic: Rate Law sign
- Replies: 3
- Views: 457
Re: Rate Law sign
Rate cannot be negative. It is defined as positive. Similarly, we usually consider speed as positive.
- Sun Mar 11, 2018 2:58 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: The Slow Step of a Reaction
- Replies: 2
- Views: 355
Re: The Slow Step of a Reaction
I think both slow step and fast step are not the overall step in the equilibrium.
The step in the equilibrium should be a combined step.
The step in the equilibrium should be a combined step.
- Sun Mar 04, 2018 5:44 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Homework Problem 15.45
- Replies: 1
- Views: 300
Re: Homework Problem 15.45
Yes we add the two steps together to get 2AC+B\rightarrow A_{2}B +2C And you can see AB appears twice but is canceled in the two steps. So AB is the intermediate substance. I think you can mark either one as the longest step for the rate determining step since the only restriction is the rate determ...
- Sun Mar 04, 2018 5:39 pm
- Forum: Zero Order Reactions
- Topic: Significance of Zero order reactions
- Replies: 3
- Views: 555
Re: Significance of Zero order reactions
zero order reaction means that the rate of the reaction is independent of the reactant concentration. So whenever the concentration of reactants changes, the rate will not change. Therefore, the rate differential law equation is Rate = k. [A] = [A]0 - kt Usually, a catalyst is present in zero order ...
- Sun Mar 04, 2018 5:26 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: kinetics an thermodynamics
- Replies: 3
- Views: 509
Re: kinetics an thermodynamics
I believe we relate kinetics and thermodynamics in entropy part where we can use enthalpy change and entropy to calculate equilibrium constant K.
However, in chapter 15, we do not talk about thermodynamics but only focus on kinetics.
However, in chapter 15, we do not talk about thermodynamics but only focus on kinetics.
- Sun Feb 25, 2018 10:38 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Midterm Q5
- Replies: 2
- Views: 515
Re: Midterm Q5
I think you can find the intact solution on the course website.
https://lavelle.chem.ucla.edu/wp-content/supporting-files/Chem14B/Midterm_exam_ans.pdf
https://lavelle.chem.ucla.edu/wp-content/supporting-files/Chem14B/Midterm_exam_ans.pdf
- Sun Feb 25, 2018 10:29 pm
- Forum: General Rate Laws
- Topic: Units for 15.3 Part B
- Replies: 4
- Views: 701
Re: Units for 15.3 Part B
I believe you do not need to put O2 in it since it is just a comment to specify the substance calculated.
- Sun Feb 25, 2018 10:05 pm
- Forum: General Rate Laws
- Topic: Slow Step
- Replies: 2
- Views: 371
Re: Slow Step
Slow step is the rate-determining step. The fast step does not affect the rate of a reaction. So when express the rate reaction of two connecting reactions, always express the reactants in the slow step.
- Sun Feb 25, 2018 9:29 pm
- Forum: General Rate Laws
- Topic: rate law for forward and backward reaction
- Replies: 2
- Views: 363
rate law for forward and backward reaction
A quick question:
Dr. Lavelle mentioned in class that we considered only forward reaction. Why would we not consider the backward reaction although some of the products will return to the reactant?
Thanks!
Dr. Lavelle mentioned in class that we considered only forward reaction. Why would we not consider the backward reaction although some of the products will return to the reactant?
Thanks!
- Sun Feb 18, 2018 11:31 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Cell potential calculation
- Replies: 4
- Views: 607
Cell potential calculation
Just a quick question.
When we calculate cell potential for a reaction composed of two half reactions. Do we always subtract the smaller standard reduction potential from the larger one? Therefore the final result is always positive?
Thank you!
When we calculate cell potential for a reaction composed of two half reactions. Do we always subtract the smaller standard reduction potential from the larger one? Therefore the final result is always positive?
Thank you!
- Sun Feb 18, 2018 8:57 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 14.13c
- Replies: 1
- Views: 290
Re: 14.13c
I think the order of substances does not matter in cell diagram. You just list the substances that participate in reaction on anode and cathode.
- Sun Feb 18, 2018 8:11 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 14.11d
- Replies: 1
- Views: 280
Re: 14.11d
Pts are just inert electrodes. The oxygen and H+ ions are on the anode side. Oxidation occurs anode side. So oxygen in the water will be oxidized into oxygen. 2H2O -->4H+ +O2+4e- On the cathode side, reduction occurs. So O2 is reduced to OH-. O2+2H2O+4e-->4OH- Then we need to balance the number of e...
- Sun Feb 18, 2018 4:56 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 14.11d
- Replies: 1
- Views: 266
Re: 14.11d
Pts are just inert electrodes. The oxygen and H+ ions are on the anode side. Oxidation occurs anode side. So oxygen in the water will be oxidized into oxygen. 2H_{2}O \rightarrow 4H^{+}+O_{2}+4e^{-} On the cathode side, reduction occurs. So O2 is reduced to OH-. O_{2}+2H_{2}O+4e^{-}\rightarrow 4OH^{...
- Sun Feb 11, 2018 9:34 pm
- Forum: Calculating Work of Expansion
- Topic: When to use this equation
- Replies: 4
- Views: 550
Re: When to use this equation
When the pressure is constant, we use w = -Pext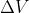
When temperature is constant (isothermal), we use w = -nRT ln (Vfinal/Vinitial)
When temperature is constant (isothermal), we use w = -nRT ln (Vfinal/Vinitial)
- Sun Feb 11, 2018 9:31 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Different ways to calculate deltaG
- Replies: 2
- Views: 405
Re: Different ways to calculate deltaG
I think the delta G of formation values in the table is just the delta G knot value. It is just the value of a substance in the standard state. However, delta G can be a value at any state, for example, not 298K or 1 atm. The result will be the same if the reaction is under the standard state. Also,...
- Sun Feb 11, 2018 9:24 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Practice Midterm #3A
- Replies: 2
- Views: 367
Re: Practice Midterm #3A
So you can first write down all the equations related to entropy. Since entropy is a state function, it can be added and substracted. \Delta S = nRln\frac{V2}{V1} \Delta S = nCln\frac{T2}{T1} \Delta S =\frac{q_{w}}{T} \Delta S = k_{b}\ln W Helium gas and Krypton are in a sealed apparatus, so the vol...
- Sun Feb 11, 2018 3:07 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs free energy and spontaneity
- Replies: 3
- Views: 474
Re: Gibbs free energy and spontaneity
Yeah, basically you need delta G to determine spontaneity.
Or you can use K to indirectly find the spontaneity at equilibrium.
Or you can use K to indirectly find the spontaneity at equilibrium.
- Sat Feb 03, 2018 11:16 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Entropy and Work
- Replies: 2
- Views: 435
Re: Entropy and Work
When T (in Kelvin) is constant, then q = -w since U = q + v.
So
Also, any energy lost by doing work is compensated by heat going into the system.Then another equation about w is
- Sat Feb 03, 2018 11:08 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 9.52
- Replies: 3
- Views: 465
Re: 9.52
Endothermic reaction means that \Delta H > 0 since it absorbs energy. The Gibb's free energy equation is \Delta G = \Delta H - T\Delta S and we know that this equation determines the spontaneity of a reaction. We want \Delta G to be negative. Now it comes to algebra. There is actually a chart to org...
- Sat Feb 03, 2018 10:59 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibb's Free Energy
- Replies: 2
- Views: 294
Re: Gibb's Free Energy
Gibb's free energy is just "G" which is a state function, but standard free energy change is \Delta G°, indicating substance in a standard state. \Delta G is the G at equilibrium since G is a quadratic function. Its tangent line at local minimum is \Delta G. And at that point, \Delta G = 0...
- Sun Jan 28, 2018 5:29 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Entropy Equations with Irreversible Paths
- Replies: 2
- Views: 245
Re: Entropy Equations with Irreversible Paths
I think the equation delta S = q/T is only for the reversible question. (for only isothermal process)
- Sun Jan 28, 2018 5:27 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Entropy [ENDORSED]
- Replies: 4
- Views: 547
Re: Entropy [ENDORSED]
Every state has a finite value for the state function entropy S(A). The difference of entropy between two states is delta S = S(B) - S(A) There are many different reversible pathways between two different states. But state function does not depend on the paths it goes from one state to another. It j...
- Sun Jan 28, 2018 5:16 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: gas with 2x positions
- Replies: 2
- Views: 409
Re: gas with 2x positions
This is set in the two-state system scenario.
When 2 particles are both in one of two states, then W = 4 = 2^2
If there is only 1 particle, then W = 2^1
So for N particles, a particle can be in one of two states, then W = 2^N
When 2 particles are both in one of two states, then W = 4 = 2^2
If there is only 1 particle, then W = 2^1
So for N particles, a particle can be in one of two states, then W = 2^N
- Sun Jan 21, 2018 6:50 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: question on solution for 8.49
- Replies: 2
- Views: 317
Re: question on solution for 8.49
If nothing is specified, the standard temperature for gas reaction is 298K.
- Sun Jan 21, 2018 6:36 pm
- Forum: Calculating Work of Expansion
- Topic: Reversible vs Irreversible
- Replies: 4
- Views: 501
Re: Reversible vs Irreversible
Reversible process: can change the reaction by changing a variable in subtle amount. The variable is usually pressure.
Also the reversible work is always greater than irreversible work.
Also the reversible work is always greater than irreversible work.
- Sun Jan 21, 2018 6:31 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Calorimetry Questions
- Replies: 2
- Views: 334
Re: Calorimetry Questions
I think we should add it. There is an example from my discussion. 20g of Cu at 45 degrees celsius is placed into 10g of water at 90 degree celsius. Find the final temperature. If the question is set in calorimeter, then q(cu)+q(cal) = -q(H2O) Because copper and calorimeter absorb heat, so the total ...
- Sun Jan 14, 2018 3:06 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Closed vs. Isolated
- Replies: 4
- Views: 613
Re: Closed vs. Isolated
You can just simply look at exercise 8.1 to help understand it. A closed system is when heat is exchangeable but not mass, for example, the mercury in a thermometer. Mercury is sealed in the thermometer, but the temperature will change with the heat supplied. So it is a closed system but not an isol...
- Sun Jan 14, 2018 2:42 pm
- Forum: Phase Changes & Related Calculations
- Topic: Exercise 8.31
- Replies: 3
- Views: 354
Exercise 8.31
Can someone explain how to do the constant pressure and volume question? 8.31 Calculate the heat released by 5.025 g of Kr(g) at 0.400 atm as it cools from 97.6 C to 25.0 C at (a) constant pressure and (b) constant volume. Assume that krypton behaves as an ideal gas. I'm really confused with these c...
- Sun Jan 14, 2018 2:32 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Infinite Forms of Hess's Law
- Replies: 5
- Views: 349
Re: Infinite Forms of Hess's Law
I think most of the formulas given are between two to four in tests since the time to finish exam is needed to be counted into consideration. However, I believe you can write infinite forms for Hess's law, especially in organic chemistry. Just consider the reactants and products and try to link them...
- Sat Dec 09, 2017 8:09 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Post Assessment 2 #24
- Replies: 2
- Views: 2472
Re: Post Assessment 2 #24
Reyna Alonso 1E wrote:bar is the atmospheric pressure at sea level so it can also be translated as as 100 kilopascals which is about 1 atm.
Thank you!
- Sat Dec 09, 2017 7:07 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Post Assessment 2 #24
- Replies: 2
- Views: 2472
Post Assessment 2 #24
24. The equilibrium constant, KP, for the reaction SO2 (g) + O2 (g) ⇌ SO3 (g) at 700 K is 3 x 104. A mixture of SO2, O2, and SO3, each at 65 bars was introduced into a container at 700 K. Is the reaction at equilibrium? If not, does SO3 tend to form or decompose? A. At equilibrium. B. Not at equilib...
- Sat Dec 09, 2017 3:20 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: The Values of K and their Limitations
- Replies: 1
- Views: 339
Re: The Values of K and their Limitations
Basically when you calculate the Kc or write Kc expression, you do not include solid and liquids as well as solvents.
And Ka, Kb and Kw also only includes aqueous solution.
Therefore the change in concentration of solids and liquids will not affect the Kc, Ka and Kb.
And Ka, Kb and Kw also only includes aqueous solution.
Therefore the change in concentration of solids and liquids will not affect the Kc, Ka and Kb.
- Sat Dec 09, 2017 11:37 am
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Midterm Question
- Replies: 2
- Views: 582
Re: Midterm Question
The point is that the uncertainty of velocity is 0.55 x2 = 1.1 m/s since it can be plus or minus 0.55. The range is actually larger. momentum(p) = m * v. uncertainty in momentum in this case = m * Δv. so Δp = 0.55 * 2 * 1.5 * 10^-3 (you need to convert grams into standard unit kilograms) The Heisenb...
- Sun Dec 03, 2017 6:08 pm
- Forum: Bronsted Acids & Bases
- Topic: 12.1
- Replies: 2
- Views: 598
Re: 12.1
I think CH3NH3+ is the best among the three you gave. Because when we want a conjugated acid of CH3NH2, you need to add one proton, in other words, one H+ to the molecule. Carbon is already connected to 3 hydrogen and then one N atom with two hydrogen asides. It is more normal to add one on N. CH3NH...
- Sun Dec 03, 2017 5:17 pm
- Forum: Lewis Acids & Bases
- Topic: Problem 12.51 Part (a)
- Replies: 3
- Views: 1128
Re: Problem 12.51 Part (a)
Actually the for acid strength: HF < HCl < HBr < HI. HCl is a stronger acid since it is completely ionized in water while HF isn't. The bond strength of HCl is weaker than HF due to the increased radius of Cl, leading to larger distance between H and Cl than H and F. So H-Cl bond is easier to break ...
- Thu Nov 30, 2017 12:02 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Part 1B Post-Module Assessment #26
- Replies: 2
- Views: 278
Part 1B Post-Module Assessment #26
26. Are solvents included in equilibrium constant expressions?
A. No
B. Yes
C. Sometimes
D. Not always
E. None of the above
I think solvents are always aqueous? but the answer is not B.
Please help me! Thanks!:)
A. No
B. Yes
C. Sometimes
D. Not always
E. None of the above
I think solvents are always aqueous? but the answer is not B.
Please help me! Thanks!:)
- Thu Nov 30, 2017 12:01 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Chem. Equilibrium Part 2 Post-Module Assess. #26 [ENDORSED]
- Replies: 2
- Views: 644
Re: Chem. Equilibrium Part 2 Post-Module Assess. #26 [ENDORSED]
I think the answer is B. N2(g) + 3H2(g) ⇌ 2NH3(g). I 0.250 0.500 0 C -0.075 -0.225 +0.15 E 0.175 0.275 0.15 Basically, the change of concentration of NH3 indicates the change of N2. If concentration of NH3 is increased by 0.15mol/L, then the N2 is decreased by 0.075mol/L, calculated by the coefficie...
- Wed Nov 29, 2017 11:28 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: From the Post-Module Assessment 1A:
- Replies: 3
- Views: 439
Re: From the Post-Module Assessment 1A:
"Lie to the right" is used when describing K at the equilibrium. It is a state. From the note, if K is small (K<10^-3), more reactants are at equilibrium. So the equilibrium sits to the left. Vice versa. This basically describes which side is favored. While "equilibrium shifts to the ...
- Sun Nov 26, 2017 9:32 pm
- Forum: Ideal Gases
- Topic: Problem 11.37 [ENDORSED]
- Replies: 3
- Views: 686
Re: Problem 11.37 [ENDORSED]
The K expression for original reaction is [NH3]^2/([H2]^3*[n2]), and the K expression for b is [NH3]/([N2]^1/2*[H2]^2/3).
Say the K expression for (b) is K2.
K is the square of K2. So if you want to get the value of K2, you need to take the square root, which is square root of 41. The answer is 6.4.
Say the K expression for (b) is K2.
K is the square of K2. So if you want to get the value of K2, you need to take the square root, which is square root of 41. The answer is 6.4.
- Sun Nov 26, 2017 9:25 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 11.39
- Replies: 2
- Views: 342
Re: 11.39
The Kc for 2BrCl + H2 = Br2 + 2HCl (you wrote Br2 instead of H2 which is wrong) is expressed as [Br2]*[HCl]^2/([H2][BrCl]^2). The Kc for 2BrCl=Br2 + Cl2 is expressed as [Br2][Cl2]/[BrCl]^2; the Kc for H2 + Cl2 = 2HCl is expressed as [HCl]^2/([H2][Cl2]). As the Kcs of later two times, you can get the...
- Sun Nov 26, 2017 9:13 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Symmetrical Molecules
- Replies: 3
- Views: 569
Re: Symmetrical Molecules
For symmetrical molecules, the polar effect can be canceled. So they are non polar molecules.
Though some molecules will have polar bonds, but if the whole molecule is symmetrical, the polar effect can be canceled out. The polar molecule such as water has an uneven distribution of electron density.
Though some molecules will have polar bonds, but if the whole molecule is symmetrical, the polar effect can be canceled out. The polar molecule such as water has an uneven distribution of electron density.
- Sat Nov 25, 2017 6:23 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Chem Equilibrium Module Question #27 & #29?
- Replies: 1
- Views: 189
Re: Chem Equilibrium Module Question #27 & #29?
I think K is the constant representing the ratio of concentration of products and reactants at equilibrium, but now initial concentrations.
For any other circumstances, we use Q to represent the ratio.
For any other circumstances, we use Q to represent the ratio.
- Sat Nov 25, 2017 6:09 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Quadratic Equations from ICE Box [ENDORSED]
- Replies: 2
- Views: 1015
Re: Quadratic Equations from ICE Box [ENDORSED]
You should choose the answer that is smaller than the initial concentration of the reactants.
Because x is just an amount of change during the reaction, so the concentration change must be smaller than the initial concentration of reactants. If it is larger, then the answer should be rejected.
Because x is just an amount of change during the reaction, so the concentration change must be smaller than the initial concentration of reactants. If it is larger, then the answer should be rejected.
- Sat Nov 25, 2017 6:00 pm
- Forum: Naming
- Topic: HW 17.29 about the metal ion [ENDORSED]
- Replies: 2
- Views: 397
HW 17.29 about the metal ion [ENDORSED]
For 17.29 b and c, I noticed that the answer for b just contains "cobalt(III) ion"; however, answer for a and c is "ferrate(II) ion" and "cobaltate(III) ion". Why is cobalt in b is written as only the metal name, but cobalt in c is written as -ate form?
Thanks!
Thanks!
- Sun Nov 19, 2017 5:35 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: HW Question 4.27
- Replies: 4
- Views: 502
Re: HW Question 4.27
Benzene is nonpolar since it has a symmetrical structure and all the bond dipoles cancel. But for pyridine, the C-H group is replaced by a nitrogen. And there is a lone pair on nitrogen. The molecule then becomes not symmetrical since C-N bond has different dipole then C-C bond. Also C-H bond dipole...
- Sun Nov 19, 2017 5:24 pm
- Forum: Hybridization
- Topic: Hybrid orbitals and bonds
- Replies: 3
- Views: 285
Re: Hybrid orbitals and bonds
the formation of sigma and pi bonds are due to orbitals interaction. Sigma bonds are formed by two orbitals end to end interact and pi bonds are formed by two p orbitals overlap side by side. For an atom in a molecule, we can determine the hybridization and number of sigma bonds and pi bonds in it. ...
- Wed Nov 08, 2017 10:05 pm
- Forum: Ionic & Covalent Bonds
- Topic: 3.25
- Replies: 6
- Views: 807
Re: 3.25
How do you know the charges for the elements? memorization? It's basically based on electron configuration, or say, how it would be most stable to form a compound. For example, for group 1 and 2, it is most possible that they are tend to lose electrons when forming a compound. Then the number of el...
- Wed Nov 08, 2017 9:48 pm
- Forum: Ionic & Covalent Bonds
- Topic: 3.77
- Replies: 2
- Views: 1304
Re: 3.77
For a the answer is HCl since the electronegativity difference is bigger between H and Cl than H and I. Cl is more electronegative than I. Also for H, you can consider it the same as Phosphorus which shares the same electronegativity. For b the answer is CF4 since F is much more electronegative than...

