Search found 17 matches
- Sun Jun 03, 2018 11:09 pm
- Forum: Ionic & Covalent Bonds
- Topic: Polarizability
- Replies: 7
- Views: 907
Re: Polarizability
What determines the size of the electron cloud?
- Sun May 20, 2018 4:27 pm
- Forum: Dipole Moments
- Topic: Methane vs Carbon tetrachloride
- Replies: 1
- Views: 281
Methane vs Carbon tetrachloride
We did this example in class but I'm still a bit confused. Why does CCl4 have a significantly higher boiling point than CH4?
- Sun May 20, 2018 3:49 pm
- Forum: Bond Lengths & Energies
- Topic: Test 3
- Replies: 8
- Views: 840
Re: Test 3
In an earlier post chem_admin mentioned test 3 would cover chapter 3, and they didn't mention any exceptions. I would go over some of the hw problems relating to bond lengths just to be safe.
- Sun May 20, 2018 3:37 pm
- Forum: Electronegativity
- Topic: Carbon and Sulfur electronegativity?
- Replies: 4
- Views: 14988
Re: Carbon and Sulfur electronegativity?
Carbon and Sulfur are actually really close in electronegativity (Carbon 2.55 and Sulfur 2.58), but as someone said earlier, using Fluorine as a reference point is a good strategy because it is the most electronegative element. According to my TA, we probably won't be asked to distinguish elements s...
- Fri May 04, 2018 11:48 am
- Forum: Quantum Numbers and The H-Atom
- Topic: Clarification on Spin Quantum Number
- Replies: 4
- Views: 523
Re: Clarification on Spin Quantum Number
I believe that magnetic spin is important because it describes the magnetic field that the electron produces. And since magnetic field can only be positive or negative that is what gives us the only two possible "spins" of an electron. Magnetic spin was actually a later discovery that help...
- Fri May 04, 2018 11:18 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: 3d orbital
- Replies: 3
- Views: 485
3d orbital
Why is it 3d and not 4d? For the other orbitals the coefficient represents the period its in, so why isn't it the same for the d orbital.
- Fri May 04, 2018 11:09 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: 4s orbital
- Replies: 2
- Views: 441
4s orbital
So a few people have already asked why 4s fills up before 3d, which I understand. What I wanted to know is why is it that the 4s orbital is lower in energy than the 3d orbital before it is filled, but is higher in energy after it is filled? Someone may have asked this already but I couldn't seem to ...
- Sat Apr 21, 2018 12:44 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Balmer Series vs Lyman Series
- Replies: 4
- Views: 1146
Re: Balmer Series vs Lyman Series
Also you should know the wavelengths for visible light are, roughly, from 400nm(violet) to 700nm(red). And as mentioned, they are a part of the Balmer Series.
- Sat Apr 21, 2018 12:35 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Quiz 2 [ENDORSED]
- Replies: 4
- Views: 417
Re: Quiz 2 [ENDORSED]
I asked some of the TAs/UAs and they said that it would be fine to use the Rydberg equation, because essentially the Rydberg formula is just a simplified equation derived from \Delta E = E_{f} - E_{i} (where E =- \frac{hR}{n^2} ). I believe the purpose of Dr. Lavelle doing it step-by-step in class w...
- Sat Apr 21, 2018 12:13 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: h-bar [ENDORSED]
- Replies: 7
- Views: 1374
Re: h-bar [ENDORSED]
I was also confused between the practicality of using 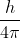 instead of
instead of 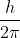 . What is the difference between the two?
. What is the difference between the two?
- Sun Apr 15, 2018 5:52 pm
- Forum: Einstein Equation
- Topic: Planck's constant [ENDORSED]
- Replies: 3
- Views: 644
Re: Planck's constant [ENDORSED]
The constant was found experimentally when Planck was studying blackbody radiation. He basically found that radiation (eg light) is emitted/absorbed in discrete energy packets (quanta). This is determined by the relationship between the frequency of radiation and Planck's constant. Here's a pretty c...
- Fri Apr 13, 2018 3:49 pm
- Forum: Properties of Electrons
- Topic: Electromagnetic Field [ENDORSED]
- Replies: 7
- Views: 860
Re: Electromagnetic Field [ENDORSED]
As other people have already said, the key takeaway is that Electric and Magnetic Fields are perpendicular, but if you wanted to learn more about why this is true you might want to look into Maxwell's Equations. I'm not sure if this course covers Maxwell, but you will learn it if you take the physic...
- Fri Apr 13, 2018 3:40 pm
- Forum: Properties of Light
- Topic: What is the speed of life though
- Replies: 3
- Views: 379
Re: What is the speed of life though
its a song by David Bowie
- Fri Apr 13, 2018 3:32 pm
- Forum: Properties of Light
- Topic: Value of speed of light [ENDORSED]
- Replies: 4
- Views: 622
Re: Value of speed of light [ENDORSED]
I believe we're supposed to use 3x10^8 m/s. On exams/tests we'll be given a sheet with all the important constants included, so don't stress too much about it.
- Sun Apr 08, 2018 3:24 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Test #1
- Replies: 1
- Views: 270
Test #1
Just need some clarification. The test will be held in discussion, correct?
- Sun Apr 08, 2018 3:20 pm
- Forum: Balancing Chemical Reactions
- Topic: How to Find Net Amount of Gas Produced? - Pre-Class Assessment [ENDORSED]
- Replies: 1
- Views: 318
Re: How to Find Net Amount of Gas Produced? - Pre-Class Assessment [ENDORSED]
The problem asks for the net production of gas, and both reactants and products are gases. So all you have to do is: (sum of coefficients from products) - (sum of coefficients from reactants) = net number of moles produced. So you should have 36-30=6 (A)
- Fri Apr 06, 2018 11:32 am
- Forum: Administrative Questions and Class Announcements
- Topic: Modules
- Replies: 5
- Views: 682
Modules
I noticed that in the "Audio-Visual Focus-Topics" instructions for the modules it says that the modules are not graded on correctness. Does that mean that they are graded on completeness? Or are they just study tools for the class that are not included in the final grade?

