Search found 30 matches
- Thu Jun 07, 2018 7:01 am
- Forum: Ionic & Covalent Bonds
- Topic: Help on 3.53
- Replies: 2
- Views: 497
Re: Help on 3.53
When you're trying to calculate the formal charge, you want to find the number of Valence electrons the atom owns, and the number of "things" that it has surrounding the atom. By "things" I mean the number of bonds and non bonded valence electrons added up together. Thus the equa...
Re: Oxidation
This website is a good reference on how to find the oxidation number.
https://socratic.org/questions/how-do-y ... a-compound
https://socratic.org/questions/how-do-y ... a-compound
- Thu Jun 07, 2018 6:57 am
- Forum: Ionic & Covalent Bonds
- Topic: Help on 3.49
- Replies: 2
- Views: 410
Re: Help on 3.49
The formal charge on the carbons is both 01
There is a triple bond between C and C, with one lone pair on each. Carbon's Valence is 4, so 4-5 (the number of bonds&valence)=-1
There is a triple bond between C and C, with one lone pair on each. Carbon's Valence is 4, so 4-5 (the number of bonds&valence)=-1
- Fri Jun 01, 2018 1:23 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angles
- Replies: 7
- Views: 849
Re: Bond Angles
I'm pretty sure we're not going to be expected to know the exceptions from repulsion (was told this in discussion), but we do need to know the main bond angles of each shape.
- Wed May 30, 2018 2:31 pm
- Forum: Hybridization
- Topic: Hybrid
- Replies: 1
- Views: 297
Re: Hybrid
Yes, regions of electron density= # of hybrid orbitals
- Tue May 29, 2018 9:10 pm
- Forum: Resonance Structures
- Topic: How to determine resonance
- Replies: 2
- Views: 471
Re: How to determine resonance
You can essentially move the bonds from top left right, etc.
- Tue May 29, 2018 9:08 pm
- Forum: Lewis Structures
- Topic: Lewis Structures and Coordinate Covalent Bonds
- Replies: 1
- Views: 437
Re: Lewis Structures and Coordinate Covalent Bonds
It can be, but since professor lavelle hasn't brought it up I wouldn't worry about it.
https://www.youtube.com/watch?v=mJppwhJvTLc
^examples
(weird intro music, stick with it)
https://www.youtube.com/watch?v=mJppwhJvTLc
^examples
(weird intro music, stick with it)
- Tue May 29, 2018 9:04 pm
- Forum: Lewis Structures
- Topic: Electrons in Valence Shell
- Replies: 4
- Views: 834
Re: Electrons in Valence Shell
I think it really just depends on the atom you're looking at. For example Sulfur has an expanded octet of twelve electrons.
This website is pretty helpful for reference.
https://opencurriculum.org/9814/what-is ... nce-shell/
This website is pretty helpful for reference.
https://opencurriculum.org/9814/what-is ... nce-shell/
- Tue May 29, 2018 9:47 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Cancelling out dipole moments
- Replies: 3
- Views: 1014
Cancelling out dipole moments
When drawing a VSEPR structure and determining if the molecule is polar or nonpolar, how do you know when dipole moments cancel?
- Thu May 24, 2018 6:49 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Ammonia shape [ENDORSED]
- Replies: 2
- Views: 745
Ammonia shape [ENDORSED]
I know we talked about this in lecture, but I am still slightly confused on why NH3 is trigonal pyramidal?
- Tue May 22, 2018 10:00 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angles
- Replies: 4
- Views: 897
Re: Bond Angles
I don't know the in-depth answer, but yes.
This website does a good job explaining it.
https://socratic.org/questions/how-does-bonding-affect-molecular-geometry
This website does a good job explaining it.
https://socratic.org/questions/how-does-bonding-affect-molecular-geometry
- Tue May 22, 2018 9:59 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Dispersion/Induced Dipole-Induced Dipole/London/Van Der Waals
- Replies: 1
- Views: 441
Re: Dispersion/Induced Dipole-Induced Dipole/London/Van Der Waals
That is the equation for the interaction potential energy - it is the energy it takes to pull the bonds (induced dipole-induced dipole, dipoles, hydrogen) apart. When two molecules come close together, the two molecules have both attractive (dipoles) and repulsive forces (electron clouds of two mole...
- Sun May 20, 2018 9:30 pm
- Forum: Lewis Structures
- Topic: chapter 3 hw #57 question
- Replies: 5
- Views: 731
Re: chapter 3 hw #57 question
You may want to reference this website, it explains it pretty well.
https://chemistry.stackexchange.com/questions/6447/why-does-clo%E2%82%84%E2%81%BB-only-have-3-double-bonds
https://chemistry.stackexchange.com/questions/6447/why-does-clo%E2%82%84%E2%81%BB-only-have-3-double-bonds
- Sun May 20, 2018 9:19 pm
- Forum: Dipole Moments
- Topic: Dipole going from positive to negative
- Replies: 1
- Views: 294
Re: Dipole going from positive to negative
The direction of dipole moment is from positive poles to negative poles because the electrons in covalent bonds are not always equally shared. When there is an increasing difference in electronegativity, it results in an increasing difference in the charge of the two atoms, increasing the ionic char...
- Sun May 20, 2018 8:32 pm
- Forum: Ionic & Covalent Bonds
- Topic: Test 3 [ENDORSED]
- Replies: 7
- Views: 1056
Re: Test 3 [ENDORSED]
We won't be expected to know the names of ionic compounds.
- Sun May 20, 2018 8:30 pm
- Forum: Ionic & Covalent Bonds
- Topic: delta positive and delta negative
- Replies: 2
- Views: 1995
Re: delta positive and delta negative
A negative dipole should be on the side that is more electronegative, and takes in electrons. It will be able to pull other electrons to it and become more negative. Thus, the positive dipole is the one that is less electronegative, giving off electrons and becoming more positive.
- Fri May 18, 2018 8:39 pm
- Forum: Dipole Moments
- Topic: Positive and Negative Dipole [ENDORSED]
- Replies: 2
- Views: 1609
Positive and Negative Dipole [ENDORSED]
I'm a bit confused on how to determine whether or not a dipole is positive or negative in an ionic or covalent bond.
- Thu May 17, 2018 7:06 am
- Forum: Ionic & Covalent Bonds
- Topic: Ionic bonds
- Replies: 6
- Views: 875
Re: Ionic bonds
I'm pretty sure we haven't entirely covered this yet in class, but in drawing ionic bonds you have to show the transfer of electrons from one atom to another. Check out this diagram and website for an example of the ionic bond between Na+ and Cl- https://chem.libretexts.org/@api/deki/files/1778/ioni...
- Thu May 17, 2018 6:52 am
- Forum: Ionic & Covalent Bonds
- Topic: How to tell which elements will be most likely to form a covalent bond
- Replies: 8
- Views: 3325
How to tell which elements will be most likely to form a covalent bond
If you're given a list of elements such as
K and Cl
H and O
Al and Mg
K and Na
how do you tell which elements will most likely form a covalent bond?
K and Cl
H and O
Al and Mg
K and Na
how do you tell which elements will most likely form a covalent bond?
- Thu May 17, 2018 6:42 am
- Forum: Bond Lengths & Energies
- Topic: exceptions
- Replies: 1
- Views: 318
Re: exceptions
Bonds between like atoms usually become weaker as we go down a column because as two bonded atoms become larger, the distance between them that is occupied by bonding electrons becomes proportionally smaller. Exceptions are single bonds between the period 2 atoms of groups 15, 16, and 17 (i.e., N, O...
- Thu May 17, 2018 6:34 am
- Forum: Lewis Structures
- Topic: HW 3.41
- Replies: 2
- Views: 358
Re: HW 3.41
That is correct, it would just be a resonance structure of ch3oh
- Sat May 12, 2018 3:13 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal charges and full valence shells
- Replies: 3
- Views: 648
Formal charges and full valence shells
What is the relationship between having full valence shells and formal charges? Do full valence shells always result in a formal charge of zero?
- Tue May 08, 2018 7:58 pm
- Forum: Trends in The Periodic Table
- Topic: electron affinity [ENDORSED]
- Replies: 15
- Views: 2025
Re: electron affinity [ENDORSED]
The electron's affinity measures the energy given off when one mole of atoms in the gaseous state each takes in one (or more) electrons to become a mole of anions in the gaseous state, or basically the energy required to gain an electron to become an anion. That being said, the nuclear charge of an ...
- Mon Apr 30, 2018 6:54 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Electrons occupying a subshell [ENDORSED]
- Replies: 1
- Views: 208
Re: Electrons occupying a subshell [ENDORSED]
Given l (the type of subshell), you can use it to find the magentic quantum number ml=-l...0...+l. This number divides the subshell into individual orbitals (s,p,d,f) which hold the electrons; there are 2l+1 orbitals in each subshell. Thus the s subshell has only one orbital, the p subshell has thre...
- Mon Apr 30, 2018 6:40 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Spin Number (Ms)
- Replies: 3
- Views: 385
Re: Spin Number (Ms)
The spin quantum number tells us the spin of the electron and is the fourth quantum number. There can only be two electrons in an orbital (Pauli's exclusion principle) and the values for ms are +1/2 and -1/2. Since electrons spin on its axis, it has both angular momentum and orbital angular momentum...
- Wed Apr 25, 2018 2:21 pm
- Forum: *Shrodinger Equation
- Topic: Quantum numbers & orbitals
- Replies: 1
- Views: 286
Quantum numbers & orbitals
Professor Lavelle said that during lecture 2s and 2p are the same in a Hydrogen atom. Why is that?
- Thu Apr 19, 2018 12:42 am
- Forum: Properties of Electrons
- Topic: Practice Problem
- Replies: 1
- Views: 314
Re: Practice Problem
Because there is zero kinetic energy given in the question, we know that the equation to use would be KE = Ep - energy needed to eject an electron (threshold). The threshold is given as 3.607 x 10-19 J and KE would be zero.
Solving algebraically for wavelength by setting Ep =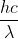
Solving algebraically for wavelength by setting Ep =
- Wed Apr 18, 2018 2:39 pm
- Forum: DeBroglie Equation
- Topic: Measurable Wavelike properties
- Replies: 3
- Views: 409
Measurable Wavelike properties
In lecture, professor Lavelle went how over solving a DeBroglie equation and asked whether or not the wavelength had measurable wavelike properties. One of the answers did while the other didn't. How did he go about solving that?
- Wed Apr 18, 2018 2:29 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Frequency if energy goes from n=2 to n=4
- Replies: 6
- Views: 603
Re: Frequency if energy goes from n=2 to n=4
The higher the energy of a photon absorbed, the higher the energy level it jumps to. Energy of a photon can not be negative.
- Wed Apr 18, 2018 2:11 pm
- Forum: DeBroglie Equation
- Topic: Homework 1.33 Part C
- Replies: 3
- Views: 387
Homework 1.33 Part C
Part C asks "what is the wavelength of the radiation that caused photoejection of the electron?" Is that referring to the wavelength of the electron that is emitted, or is it talking about the energy that is needed to eject the electron? Could you just use the E=hc/wavelength formula or wo...

