Search found 32 matches
- Fri Jun 08, 2018 10:24 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: coordination number=polydentate number?
- Replies: 5
- Views: 603
Re: coordination number=polydentate number?
Thanks guys, I think that if there are multiple ligands on the metal, though, that the polydentate number of each ligand does not necessarily equal the coordination number of the whole complex. I definitely have to think about this more.
- Thu Jun 07, 2018 7:46 pm
- Forum: Calculating the pH of Salt Solutions
- Topic: How temperature affects pH
- Replies: 3
- Views: 2583
How temperature affects pH
Why and how does temperature affect pH?
- Thu Jun 07, 2018 7:42 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: coordination number=polydentate number?
- Replies: 5
- Views: 603
coordination number=polydentate number?
Does the number of coordination bonds in a coordination compound always equal the number of bonds that a ligand makes with the central metal atom (polydentate number)?
- Thu May 31, 2018 6:15 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Double bond
- Replies: 3
- Views: 499
Re: Double bond
Hi Melissa,
So pi bonds do have overlaps in 2 places, but these are considered just 2 regions of 1 bond. I guess this is because there are only 2 electrons involved (1 from one p and 1 from the other p).
So pi bonds do have overlaps in 2 places, but these are considered just 2 regions of 1 bond. I guess this is because there are only 2 electrons involved (1 from one p and 1 from the other p).
- Thu May 31, 2018 6:08 pm
- Forum: Hybridization
- Topic: Hybridization
- Replies: 2
- Views: 453
Re: Hybridization
To add on, the "correct" number of hybridization orbitals is a consequence of the atom reaching minimal energy when it forms a bond. Atomic orbitals hybridize when the atom is bonding to other atoms, so that the atom can be less energetic.
- Thu May 31, 2018 5:58 pm
- Forum: Hybridization
- Topic: Why # regions of e density= # atomic orbitals?
- Replies: 3
- Views: 518
Re: Why # regions of e density= # atomic orbitals?
Thanks Beverly! This sentence "The 2p3 shell is a mix of the 2s shell (1 orbital) and 2p shell (3 orbitals) so it has 4 hybrid orbitals." was particularly helpful. More specifically, I'm wondering why there are necessarily 4 ways (hybrid orbitals) to mix 4 atomic orbitals.
- Thu May 31, 2018 5:31 pm
- Forum: Hybridization
- Topic: Why # regions of e density= # atomic orbitals?
- Replies: 3
- Views: 518
Why # regions of e density= # atomic orbitals?
I was interested in this fact from Wednesday's lecture. The number of atomic orbitals in an atom is the same as the number of regions of electron density that appear when the atom participates in bonding. Is this just a really cool coincidence? Why is this? A key part in my question is about why the...
- Fri May 25, 2018 10:48 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizing power
- Replies: 10
- Views: 8756
Re: Polarizing power
Also, small and highly charged cations have high polarizing power while large anions have high polarizability (distorted easily).
- Thu May 24, 2018 6:21 pm
- Forum: Ionic & Covalent Bonds
- Topic: Polar Vs. Non-Polar
- Replies: 7
- Views: 1364
Re: Polar Vs. Non-Polar
The difference in electronegativity of the atoms involved in the bond determines polarity. Difference in electronegativity means the electrons like to hang out closer to one atom than to the other, and this is called polar. When there is no difference in electronegativity (bond between atoms of the ...
- Sun May 20, 2018 11:49 am
- Forum: Dipole Moments
- Topic: Why fluctuations? [ENDORSED]
- Replies: 2
- Views: 357
Re: Why fluctuations? [ENDORSED]
Oh that makes sense. When molecules move around they change where attractions and repulsions are located. This changes the orientation of dipoles and causes constant fluctuation. I think I get it, thanks!
- Sun May 20, 2018 11:39 am
- Forum: Octet Exceptions
- Topic: Empty d-orbital?
- Replies: 2
- Views: 749
Empty d-orbital?
I'm a bit confused about this phrase "empty d-orbital", because earlier in the course I got the sense that orbitals are states that electrons can be in, rather than locations. My understanding is that when an atom has no electrons in a d-orbital state, then the d-orbital does not exist for...
- Sun May 20, 2018 11:32 am
- Forum: Dipole Moments
- Topic: Why fluctuations? [ENDORSED]
- Replies: 2
- Views: 357
Why fluctuations? [ENDORSED]
In induced dipole-induced dipole interactions, fluctuations in electron probability cloud density in one molecule causes complementary fluctuations in a nearby molecule. Why are there fluctuations in the electron cloud to begin with, and why doesn't the system equilibrate until both molecules are st...
- Sun May 20, 2018 11:24 am
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Trends of Polarizing Power
- Replies: 4
- Views: 13984
Re: Trends of Polarizing Power
Is it true that cations and anions have opposite polarizability trends?
- Sun May 20, 2018 11:20 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Polarizability and boiling point
- Replies: 2
- Views: 3987
Re: Polarizability and boiling point
Hi,
High polarizability means more covalent character,which means tighter bonding, and therefore means high boiling point. Please correct me if I'm wrong, I'm not quite sure either.
High polarizability means more covalent character,which means tighter bonding, and therefore means high boiling point. Please correct me if I'm wrong, I'm not quite sure either.
- Sun May 13, 2018 10:40 am
- Forum: Lewis Structures
- Topic: Multiple Central atoms
- Replies: 1
- Views: 320
Re: Multiple Central atoms
It is possible to have multiple central atoms. Though I'm not sure how you would go about making the lewis structure for that kind of molecule.
- Sun May 13, 2018 10:35 am
- Forum: Lewis Structures
- Topic: Lewis Structures
- Replies: 4
- Views: 591
Re: Lewis Structures
I'm not sure. I think as long as the formal charges of all the atoms in your structure are as close as possible to zero, you're fine.
- Sun May 13, 2018 10:34 am
- Forum: Ionic & Covalent Bonds
- Topic: 3.33
- Replies: 5
- Views: 632
Re: 3.33
Hi,
Ionization energy increases across periods and decreases down columns. Keep this in mind when deciding which element has the lowest ionization energy (it will be closer to the lower left corner of the periodic table).
Ionization energy increases across periods and decreases down columns. Keep this in mind when deciding which element has the lowest ionization energy (it will be closer to the lower left corner of the periodic table).
- Fri May 04, 2018 9:28 pm
- Forum: Properties of Electrons
- Topic: Exciting Electrons
- Replies: 2
- Views: 518
Re: Exciting Electrons
Yes, and if the electrons are being added to the same shell (as in moving across the periodic table) then the radius of the atom actually decreases because the electrons are being pulled in by increased effective nuclear charge.
- Fri May 04, 2018 8:42 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: 4s orbital
- Replies: 2
- Views: 440
Re: 4s orbital
So to my understanding, 4s is lower in energy than 3d for s-block elements because the s orbital shape is a sphere and is "simpler" or lower in energy than the complicated energetic shape of the p orbital. 4s electrons are removed first though, because they are farther from the nucleus tha...
- Fri May 04, 2018 8:35 pm
- Forum: DeBroglie Equation
- Topic: Bohr vs Speed of Light Equation
- Replies: 3
- Views: 1234
Re: Bohr vs Speed of Light Equation
Hi,
Both are good, it just depends on what information you are given. Typically, questions about the relationship between wavelength and frequency are best solved with c=lambda*nu. And questions about the energy of a photon are best solved with E=h*nu.
Both are good, it just depends on what information you are given. Typically, questions about the relationship between wavelength and frequency are best solved with c=lambda*nu. And questions about the energy of a photon are best solved with E=h*nu.
- Sat Apr 28, 2018 5:58 pm
- Forum: Photoelectric Effect
- Topic: What is work function? [ENDORSED]
- Replies: 15
- Views: 5728
Re: What is work function? [ENDORSED]
An important point is that it is the amt. of energy required to remove an electron from an atom such that it comes off with zero kinetic energy. There's no energy left over to move the electron away from the atom, only enough to detach it.
- Sat Apr 28, 2018 5:49 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: 1.43 [ENDORSED]
- Replies: 1
- Views: 222
Re: 1.43 [ENDORSED]
The second part just means that the uncertainty in the electron's position is the diameter of the atom. In other words, the electron can occupy any position along this imaginary 350 pm long 1D box (which is a line). The book uses this box analogy that's kind of confusing, but that's what it means fo...
- Sat Apr 28, 2018 5:43 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: What do l and m represent? [ENDORSED]
- Replies: 6
- Views: 1092
Re: What do l and m represent? [ENDORSED]
l is the angular momentum quantum number and it determines the shape of the orbital. m is the magnetic quantum number and it determines the orientation of the orbital.
- Fri Apr 20, 2018 7:20 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Rydberg’s formula
- Replies: 1
- Views: 272
Re: Rydberg’s formula
What Professor Lavelle did was the exact same thing as the Rydberg equation. He took the difference in energies at the two levels. If you look at the Rydberg equation, you'll see that that's what it does. The constants look different just because they're rearranged, but they are the same. I recommen...
- Fri Apr 20, 2018 7:12 pm
- Forum: DeBroglie Equation
- Topic: Photons? [ENDORSED]
- Replies: 4
- Views: 566
Re: Photons? [ENDORSED]
I'm unclear on why E=h/p can't be used for photons. After all, photons do have momentum p and E=h/p is derived from equations that describe photons, so why doesn't E=h/p also describe photons? Photons don't have mass but they do have momentum, so I don't see why E=h/p can't be used for photons. I'm ...
- Fri Apr 20, 2018 7:04 pm
- Forum: Photoelectric Effect
- Topic: Plastic vs Metal [ENDORSED]
- Replies: 3
- Views: 497
Plastic vs Metal [ENDORSED]
We discussed this in class today, but I'm still unsure of the reason why plastic can't be used for the experiment. Is it because the experimental setup requires a conductive material, or is it because plastics (and other non-conductive materials) don't emit photons? thanks
- Sat Apr 14, 2018 9:45 am
- Forum: Properties of Electrons
- Topic: Electromagnetic Field [ENDORSED]
- Replies: 7
- Views: 857
Re: Electromagnetic Field [ENDORSED]
The E-M fields are perpendicular to each other always while they oscillate. The electric field can oscillate up and down if the magnetic field oscillates right and left. These oscillations form the light wave.
- Sat Apr 14, 2018 9:33 am
- Forum: *Black Body Radiation
- Topic: Black Body? [ENDORSED]
- Replies: 13
- Views: 2147
Re: Black Body? [ENDORSED]
Fun fact related to this topic: the Sun is a really efficient black body.
- Sat Apr 14, 2018 9:23 am
- Forum: Photoelectric Effect
- Topic: Post module quiz #25
- Replies: 6
- Views: 338
Re: Post module quiz #25
I also think the answer is D, because it directly relates energy E to frequency 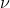 .
.
- Fri Apr 06, 2018 5:17 pm
- Forum: Limiting Reactant Calculations
- Topic: Module 3: Limiting Reactant Calculations Problem 21 [ENDORSED]
- Replies: 2
- Views: 245
Re: Module 3: Limiting Reactant Calculations Problem 21 [ENDORSED]
Hi, I think you forgot to balance the equation. With the balanced equation, the limiting reactant will be the same as what you found, but you will have a different molar ratio to work with to find the mass of AgCl.
- Fri Apr 06, 2018 4:56 pm
- Forum: Balancing Chemical Reactions
- Topic: Subscripts [ENDORSED]
- Replies: 6
- Views: 917
Re: Subscripts [ENDORSED]
Remember that subscripts indicate that the atoms are bonded to form a molecule while coefficients just count the molecules. For example, 6C4 represents 6 molecules (or moles) of C4.
- Fri Apr 06, 2018 4:42 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Why does carbon have molar mass 12.011g/mol? [ENDORSED]
- Replies: 1
- Views: 347
Why does carbon have molar mass 12.011g/mol? [ENDORSED]
Hello!
The definition of a mole is the number of atoms in exactly 12 g of carbon, and it follows that the mass of 1 mole of carbon should be exactly 12g. So, I'm confused about why the molar mass of carbon is 12.011 g/mol. Could anyone help explain this to me?
Thanks,
Mei
The definition of a mole is the number of atoms in exactly 12 g of carbon, and it follows that the mass of 1 mole of carbon should be exactly 12g. So, I'm confused about why the molar mass of carbon is 12.011 g/mol. Could anyone help explain this to me?
Thanks,
Mei

