This helps so much!!! Thank you :)
One quick question though, how do you know to tell the difference between a weak and strong acid/base based on its formula alone?
Search found 30 matches
- Thu Jun 14, 2018 12:39 am
- Forum: Bronsted Acids & Bases
- Topic: Acid-Base Reactions and Ionic Equations
- Replies: 2
- Views: 1235
- Wed Jun 13, 2018 11:34 pm
- Forum: Naming
- Topic: Oxidation Numbers (Final) [ENDORSED]
- Replies: 1
- Views: 598
Oxidation Numbers (Final) [ENDORSED]
Will we be given the oxidation numbers for the final?
- Fri Jun 08, 2018 1:40 am
- Forum: Bronsted Acids & Bases
- Topic: J.7 Select Acids and Bases
- Replies: 1
- Views: 551
J.7 Select Acids and Bases
Select an acid and a base for a neutralization reaction that results in the formation of: a) potassium bromide b) zinc nitrite c) calcium cyanide d) potassium phosphate Write the balanced equation for each reaction. How do I begin this problem? How do I determine whether an element (for instance, z...
- Fri Jun 08, 2018 1:05 am
- Forum: Bronsted Acids & Bases
- Topic: J.5
- Replies: 2
- Views: 449
Re: J.5
Where did you see the 2H2O? I only saw H2O in the answer
- Fri Jun 08, 2018 1:02 am
- Forum: Bronsted Acids & Bases
- Topic: Acid-Base Reactions and Ionic Equations
- Replies: 2
- Views: 1235
Acid-Base Reactions and Ionic Equations
Can someone please explain to me how to start the process of writing the overall, complete ionic, and net ionic equations for acid-base reactions? I'm still really confused about how to do it all. Here's an example problem that I kinda know how to do but maybe it would help with explaining the steps...
- Fri Jun 08, 2018 12:47 am
- Forum: Bronsted Acids & Bases
- Topic: J.5a&b
- Replies: 1
- Views: 486
J.5a&b
J.5a HF(aq) + NaOH(aq) -> NaF(aq) + H2O(l) -Why is the complete ionic equation for this problem HF + Na+ + OH- -> Na+ + F- + H2O and not H+ + F- + Na+ + OH- -> Na+ + F- + H2O ? J.5b (CH3)3N(aq) + HNO3(aq) -> (CH3)N3HNO3(aq) -I don't understand why (CH3)3N(aq) + HNO3(aq) just combines to become (CH3...
- Fri Jun 01, 2018 10:00 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 4.3 Structure and Shape [ENDORSED]
- Replies: 4
- Views: 547
4.3 Structure and Shape [ENDORSED]
When it asks us to draw the structure of molecules so that we can determine its molecular shape, do we draw it as a ball-and-stick diagram or do we use a Lewis structure? I know the answer in the back of the book is in the form of a Lewis diagram but I just wanted to clarify.
- Fri May 25, 2018 10:51 am
- Forum: Ionic & Covalent Bonds
- Topic: ionic and covalent character
- Replies: 7
- Views: 3151
Re: ionic and covalent character
Heteronuclear atoms with an electronegativity difference: -of >2 are considered mainly ionic bonds -of <1.5 are considered mainly covalent bonds What about the range inbetween? Is it difficult to tell when the number is between 1.5 and 2 (acts as a gray area and determining bonds depends on a host o...
- Fri May 25, 2018 10:32 am
- Forum: Octet Exceptions
- Topic: 3.61
- Replies: 4
- Views: 4683
3.61
Can someone please explain to me why the Lewis structure of ICl4^-1 looks like this?
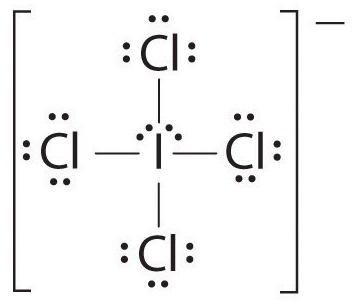
How is it determined that there are 2 lone pairs surrounding iodine?

How is it determined that there are 2 lone pairs surrounding iodine?
- Fri May 25, 2018 10:27 am
- Forum: Lewis Structures
- Topic: Multiple Compounds (3.41)
- Replies: 3
- Views: 441
Re: Multiple Compounds (3.41)
Okay, cool, thanks so much!
- Fri May 25, 2018 9:55 am
- Forum: Resonance Structures
- Topic: ranking
- Replies: 11
- Views: 1516
Re: ranking
If the two resonance structures have the same formal charge, would they be considered equal in rank?
- Fri May 25, 2018 9:20 am
- Forum: Lewis Structures
- Topic: Multiple Compounds (3.41)
- Replies: 3
- Views: 441
Multiple Compounds (3.41)
How would I start the process of drawing a Lewis structure with multiple compounds?
For instance, 3.41b asks us to draw the structure for CH3OH. How would I begin? Same goes for 3.41c: H2C(NH2)COOH
For instance, 3.41b asks us to draw the structure for CH3OH. How would I begin? Same goes for 3.41c: H2C(NH2)COOH
- Fri May 25, 2018 8:46 am
- Forum: Electronegativity
- Topic: electronegativity chart
- Replies: 11
- Views: 1879
Re: electronegativity chart
You can also remember "FONCL" from class as it describes the trend in electronegativity--as you go from F to L, electronegativity decreases.
- Wed May 09, 2018 9:23 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: 1.23 Homework
- Replies: 2
- Views: 387
Re: 1.23 Homework
Oh okay I see, thanks!
- Wed May 09, 2018 9:21 pm
- Forum: Empirical & Molecular Formulas
- Topic: Question 3 on midterm [ENDORSED]
- Replies: 8
- Views: 1209
Re: Question 3 on midterm [ENDORSED]
Honestly I took a bunch of random steps and hoped it worked out. I started with the molar masses to figure out the moles being used during the combustion. Because we know that ~0.3 grams of nicotine were burned and what the products were, I calculated how many moles of nicotine was in the 0.3 sample...
- Wed May 09, 2018 3:24 pm
- Forum: SI Units, Unit Conversions
- Topic: units
- Replies: 4
- Views: 664
Re: units
Use the following SI units:
-meter (m) for length
-kilogram (kg) for mass
-second (s) for time
-Kelvin (K) for temperature
There are only 7 SI units I believe and every other unit is derived from these. I only listed the ones I found relevant for this test.
-meter (m) for length
-kilogram (kg) for mass
-second (s) for time
-Kelvin (K) for temperature
There are only 7 SI units I believe and every other unit is derived from these. I only listed the ones I found relevant for this test.
- Wed May 09, 2018 1:49 pm
- Forum: Properties of Light
- Topic: Diffraction Patterns
- Replies: 3
- Views: 817
Re: Diffraction Patterns
Here's a really good Crash Course video that helps conceptualize diffraction and its significance in Quantum Theory:
https://youtu.be/IRBfpBPELmE
It'll help visualize the constructive and destructive waves as well.
https://youtu.be/IRBfpBPELmE
It'll help visualize the constructive and destructive waves as well.
- Wed May 09, 2018 10:06 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: 1.23 Homework
- Replies: 2
- Views: 387
1.23 Homework
The y-ray photons emitted by the nuclear decay of a technetium-99 atom used in pharmaceuticals have an energy of 140.511 keV. Calculate the wavelength of these y-rays.
I managed to convert the keV to Joules but now I don't know what to do. Can someone please help?
I managed to convert the keV to Joules but now I don't know what to do. Can someone please help?
- Wed May 09, 2018 1:41 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: 2.45
- Replies: 1
- Views: 343
2.45
Which elements are predicted to have the following ground-state electron configurations:
(d) [Rn] 7s^2 6d^2
Why is the answer Th (thorium)? I know that the d orbital comes before f, but isn't thorium an actinoid (therefore belonging in the f-block)? I'm really confused, please help!
(d) [Rn] 7s^2 6d^2
Why is the answer Th (thorium)? I know that the d orbital comes before f, but isn't thorium an actinoid (therefore belonging in the f-block)? I'm really confused, please help!
- Wed May 09, 2018 12:49 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: HW 2.29 [ENDORSED]
- Replies: 4
- Views: 642
Re: HW 2.29 [ENDORSED]
I understand why a = 6 electrons and why b/d = 2 electrons, but why is c = 8 electrons?
- Wed May 09, 2018 12:29 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Shells?
- Replies: 7
- Views: 937
Re: Shells?
So are shells and energy levels interchangeable?
- Wed May 09, 2018 12:21 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Clarification on magnetic quantum number
- Replies: 6
- Views: 837
Re: Clarification on magnetic quantum number
Could anyone help me figure out the reason WHY the values of ml go from "-l" to "l" though? I feel like I'm still conceptually confused.
- Mon May 07, 2018 12:21 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Stoichiometric Coefficient vs Moles
- Replies: 1
- Views: 278
Stoichiometric Coefficient vs Moles
Does the stoichiometric coefficient of a substance indicate its number of moles?
For instance, is 2H2O equivalent to 2 moles of H2O?
For instance, is 2H2O equivalent to 2 moles of H2O?
- Mon May 07, 2018 12:12 am
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Coulomb Potential Energy [ENDORSED]
- Replies: 1
- Views: 432
Coulomb Potential Energy [ENDORSED]
I'm still a little confused about what Coulomb's Potential Energy is. Could someone please explain its significance and whether or not we need to thoroughly understand it for the midterm?
- Mon Apr 23, 2018 1:12 pm
- Forum: Properties of Light
- Topic: Electromagnetic Spectrum [ENDORSED]
- Replies: 7
- Views: 786
Re: Electromagnetic Spectrum [ENDORSED]
To help memorize the electromagnetic spectrum I generally start from the middle of the spectrum. Visible light ranges from ~400nm to ~700nm. Because waves at 400nm emit VIOLET light, waves a bit below that number are ultraVIOLET. Then, because waves at 700nm emit RED light, waves a bit above that nu...
- Mon Apr 23, 2018 1:05 pm
- Forum: Properties of Light
- Topic: Difference between wave model and particle model? [ENDORSED]
- Replies: 6
- Views: 15069
Re: Difference between wave model and particle model? [ENDORSED]
Regarding intensity, its properties vary according to which model you're looking at. In the wave model, the intensity of the radiation is proportional to the square amplitude of the wave. In the particle model, the intensity of the radiation is proportional to the number of photons present at each i...
- Mon Apr 23, 2018 1:03 pm
- Forum: Properties of Light
- Topic: Amplitude and Intensity
- Replies: 6
- Views: 712
Re: Amplitude and Intensity
When referring to light in the aspect of the wave-particle duality, the intensity of the light and its properties vary according to which model you're looking at. In the wave model, the intensity of the radiation is proportional to the square amplitude of the wave. In the particle model, the intensi...
- Mon Apr 23, 2018 1:00 pm
- Forum: Properties of Light
- Topic: photoelectric effect
- Replies: 4
- Views: 520
Re: photoelectric effect
The "surprise" was that Planck proposed energy was transferred in discrete packets (photons) which discarded classical physics. In classical physics, there is no restriction on how small an amount of energy may be transferred from one object to another. However, quantum mechanics focuses o...
- Mon Apr 23, 2018 1:41 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Wave and Particle Properties of Electrons and Photons
- Replies: 4
- Views: 218
Re: Wave and Particle Properties of Electrons and Photons
How does constructive/destructive interference relate to the wave-particle duality then? I'm still a little confused
- Mon Apr 23, 2018 12:43 am
- Forum: *Black Body Radiation
- Topic: Black Body? [ENDORSED]
- Replies: 13
- Views: 2147
Re: Black Body? [ENDORSED]
Could someone please explain the relationship between the ultraviolet catastrophe and black bodies then?

