I think you are trying to calculate the strength of the same type of acid (eg: HCl) before and after dilution. Since pH value is affected by the [H+], calculating the concentration of H+ in both solutions can give you the pH value by using
pH = -log[H+]
Search found 30 matches
- Fri Jun 08, 2018 1:34 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Calculating the difference between two pH values
- Replies: 2
- Views: 513
- Fri Jun 08, 2018 1:27 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Charge of ligands
- Replies: 5
- Views: 729
Charge of ligands
Since there is a large number of ligands that form coordination compounds with metallic atoms, do we have to memorize the charges of these ligands or is it more beneficial to determine the charge using Lewis structure?
- Thu May 31, 2018 4:51 pm
- Forum: Hybridization
- Topic: How to identify pi and sigma bonds
- Replies: 4
- Views: 759
Re: How to identify pi and sigma bonds
It is important to remember that the sigma bond will always form first, followed by the pi bonds. In the formation of multiple bonds, there will always be ONE sigma bond followed by 1 or 2 pi bonds.
- Thu May 31, 2018 4:46 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Define Ligands?
- Replies: 10
- Views: 1164
Re: Define Ligands?
Do we only classify certain molecules as ligands when it is involved in the formation of an organometallic complex?
- Thu May 31, 2018 4:26 pm
- Forum: Sigma & Pi Bonds
- Topic: Double bond with 2 pi bonds?
- Replies: 2
- Views: 454
Re: Double bond with 2 pi bonds?
A sigma bond will always be formed first during the formation of a double bond so a double bond with 2 pi bonds is highly unlikely.
- Thu May 31, 2018 4:19 pm
- Forum: Hybridization
- Topic: sp^3 orbitals
- Replies: 3
- Views: 411
Re: sp^3 orbitals
Hi Adrienne,
I think instead of unfilled sp3 orbitals, it should be unpaired electron in sp3 orbitals that form σ-bonds since empty orbitals are not able to form any bonds with other atoms
I think instead of unfilled sp3 orbitals, it should be unpaired electron in sp3 orbitals that form σ-bonds since empty orbitals are not able to form any bonds with other atoms
- Wed May 23, 2018 9:20 pm
- Forum: Lewis Structures
- Topic: 3.35
- Replies: 1
- Views: 388
Re: 3.35
Ionic compounds can be made up of covalent bonds, and will only form ionic bonds when the compounds are bonded to another ionic compound.
- Wed May 23, 2018 7:57 pm
- Forum: Lewis Structures
- Topic: Finding the central element on the Lewis structure
- Replies: 4
- Views: 1695
Re: Finding the central element on the Lewis structure
Does this mean if the compound is made up of group 1 or 2 elements and the FONCl elements, the metal elements will always be the central element?
- Fri May 18, 2018 11:51 am
- Forum: Ionic & Covalent Bonds
- Topic: Solubility
- Replies: 3
- Views: 479
Re: Solubility
Compounds with covalent bond do not dissolve but dissolve in other organic solvents. Ionic compounds dissolve in water as the ions dissociate in the presence of H+ and OH- ions in water
- Fri May 18, 2018 11:47 am
- Forum: Dipole Moments
- Topic: Defining "Distortion"
- Replies: 1
- Views: 2492
Re: Defining "Distortion"
Polarizing power refers to the ability of a cation to distort the electron cloud of the anion in an ionic compound. The electron cloud of an anion is pulled towards the cation when it is distorted, giving the ionic bond a covalent character. If the polarization is quite small, an ionic bond is forme...
- Wed May 16, 2018 12:33 pm
- Forum: Lewis Structures
- Topic: formal charge on central atom
- Replies: 3
- Views: 512
Re: formal charge on central atom
Does the formal charge of the central atom affect the overall charge of the molecule?
- Wed May 16, 2018 12:31 pm
- Forum: Ionic & Covalent Bonds
- Topic: Lewis Acids and Bases
- Replies: 4
- Views: 526
Re: Lewis Acids and Bases
In order to determine the strength (bond strength) of an acid, it depends on the size of the 'A' atom: the smaller the 'A' atom, the stronger the H-A bond. When going down a row in the Periodic Table, the atoms get larger so the strength of the bonds get weaker, which means the acids get stronger. F...
- Thu May 10, 2018 8:15 pm
- Forum: Ionic & Covalent Bonds
- Topic: Difference between anions and cations?
- Replies: 3
- Views: 1691
Re: Difference between anions and cations?
Anions: when an atom gains electrons and form a negatively charged ion due to the increased number of electron
Cations: when an atom loses electrons and form a positively charged ion due to the decreased number of electron
This is basically how I remember it so I hope it helps!
Cations: when an atom loses electrons and form a positively charged ion due to the decreased number of electron
This is basically how I remember it so I hope it helps!
- Thu May 10, 2018 8:06 pm
- Forum: Ionic & Covalent Bonds
- Topic: Octet
- Replies: 7
- Views: 1016
Re: Octet
Since the first four elements will not achieve octet, is there a special term for the condition when these elements achieve stable state and all the orbitals are filled?
- Wed May 09, 2018 4:09 pm
- Forum: Ionic & Covalent Bonds
- Topic: Midterm Topics [ENDORSED]
- Replies: 33
- Views: 4923
Re: Midterm [ENDORSED]
Maldonado3K wrote:Hello there, I am overthinking everything.. but just to confirm.. our midterm is taking place in CS50 correct? Thanks!
It depends on your last name
A-F at CS24
G-N at Franz 1178
O-Z at CS76
Hope this helps!
- Wed May 09, 2018 11:02 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: q16
- Replies: 2
- Views: 394
Re: q16
hi can you clarify which question 16 because I would love to help!
- Sat May 05, 2018 3:07 pm
- Forum: Trends in The Periodic Table
- Topic: Ionic radius trend
- Replies: 2
- Views: 404
Re: Ionic radius trend
During the formation of a cation, electrons are lost from their shells and since the radius is directly affected by the number of shells, this causes the ion to possess less number of shells than their parent atom, hence they are smaller. Meanwhile, during the formation of an anion, electrons are ga...
- Sat May 05, 2018 2:45 pm
- Forum: Limiting Reactant Calculations
- Topic: Homework M12
- Replies: 3
- Views: 576
Re: Homework M12
Based on the calculations, we can find the masses of the products, Fe and Al_2O_3 which are 4.907g and 4.4814 g respectively. Therefore, the mass of excess reactant leftover can be calculated by subtracting the total mass of products from the initial total mass of reactants. Mass of excess reactants...
- Sat May 05, 2018 2:07 pm
- Forum: Balancing Chemical Reactions
- Topic: Test 1 Q3
- Replies: 2
- Views: 454
Re: Test 1 Q3
Answer: _2 + 2NO +4H_2O)
Usually, for balancing equation questions, I try balancing the least abundant element first which is Cu in this case. Hope this helps!
Usually, for balancing equation questions, I try balancing the least abundant element first which is Cu in this case. Hope this helps!
- Fri Apr 27, 2018 1:19 pm
- Forum: Properties of Light
- Topic: Energy difference in excited electrons
- Replies: 3
- Views: 384
Energy difference in excited electrons
I understand that we can use the Rydberg equation to calculate the energy change when electrons are excited from one energy level to another. But what should we do when the values of n are not given? Do we use the Lyman and Balmer series value of n1 as the original value of n?
- Fri Apr 27, 2018 1:05 pm
- Forum: Balancing Chemical Reactions
- Topic: balancing chemical reactions
- Replies: 7
- Views: 2579
Re: balancing chemical reactions
Cu + 4HNO3 --> Cu(NO3)2 + 2NO + 2 H2O
- Thu Apr 26, 2018 4:47 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: higher energy/ lower energy
- Replies: 2
- Views: 312
Re: higher energy/ lower energy
S, p, d and f are orbitals for the electrons. Electrons in s orbital has lower energy as it is the smallest orbital and this the energy of the electrons increases when it is in larger orbitals. Size of orbital: s<p<d<f.
- Fri Apr 20, 2018 1:37 pm
- Forum: Photoelectric Effect
- Topic: Threshold Energy [ENDORSED]
- Replies: 12
- Views: 1820
Re: Threshold Energy [ENDORSED]
What affects the threshold energy of a photon?
- Fri Apr 20, 2018 1:34 pm
- Forum: Photoelectric Effect
- Topic: Post-Assessment #31 [ENDORSED]
- Replies: 2
- Views: 385
Re: Post-Assessment #31 [ENDORSED]
If you are calculating the kinetic energy of the ejected electron, you can use the equation 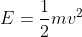
where m=mass of an electron and v=velocity of the electron after it's ejected
where m=mass of an electron and v=velocity of the electron after it's ejected
- Fri Apr 20, 2018 1:28 pm
- Forum: Properties of Light
- Topic: Wavelengths
- Replies: 8
- Views: 697
Re: Wavelengths
I think it also helps if we know the order of frequency of the electromagnetic waves, which is the inverse of the wavelength order.
- Thu Apr 19, 2018 3:56 pm
- Forum: Properties of Electrons
- Topic: Geiger -Marsden experiment [ENDORSED]
- Replies: 5
- Views: 646
Re: Geiger -Marsden experiment [ENDORSED]
Do we have to study the experiment in detail or do we just have to know the concepts and result of the experiment?
- Thu Apr 12, 2018 4:50 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: When to use MiVi=MfVf
- Replies: 8
- Views: 4445
Re: When to use MiVi=MfVf
Hello, I'm having some difficulty determining when to use the M(initial) x V(initial) = M(final) x V(final) equation in problems. Is there any way to know to use this equation in a type of problem? What kind of problems is it used for? Thanks! You can use this equation to find either molarity/conce...
- Thu Apr 12, 2018 4:41 pm
- Forum: Properties of Electrons
- Topic: Electromagnetic Field [ENDORSED]
- Replies: 7
- Views: 857
Re: Electromagnetic Field [ENDORSED]
I think the direction of the waves can be interchangeable as long as they are drawn perpendicular to each other.
- Thu Apr 12, 2018 4:39 pm
- Forum: SI Units, Unit Conversions
- Topic: Sig Figs
- Replies: 9
- Views: 1097
Re: Sig Figs
In case of calculations that require the usage of values from the periodic table, are we supposed to use the exact value stated or should we round it up?
- Sat Apr 07, 2018 8:58 pm
- Forum: Significant Figures
- Topic: All students read this sig fig post [ENDORSED]
- Replies: 170
- Views: 35146
Re: All students read this sig fig post [ENDORSED]
I am wondering how many sig figs are we supposed to round off our answer to after getting the value from a calculator?

