Search found 34 matches
- Sun Jun 10, 2018 8:27 pm
- Forum: Properties of Electrons
- Topic: Wave-like Properties of Electron
- Replies: 5
- Views: 674
Wave-like Properties of Electron
How can you tell whether the wavelike properties of ejected electrons can be detected? Is there a certain numerical cutoff?
- Sat Jun 09, 2018 8:54 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Chapter4 Question7C
- Replies: 1
- Views: 342
Re: Chapter4 Question7C
Well, with trigonal pyramidal shape, the bond angles are 109.5 if there are no lone pairs on the central atom. If there is a lone pair(s), then they push the non-central atoms closer together (repulsion). Since they are pushed together, they are going to have a bond angle of less than 109.5, 107 if ...
- Sat Jun 09, 2018 8:31 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Molar Mass
- Replies: 5
- Views: 854
Molar Mass
Hi,
When converting from grams to moles of say,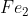 or
or 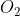 would the grams per mole value be 16 (1 oxygen), or 32 (2 oxygen)?
would the grams per mole value be 16 (1 oxygen), or 32 (2 oxygen)?
When converting from grams to moles of say,
- Fri Jun 08, 2018 9:11 am
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: How to tell acid from base?
- Replies: 1
- Views: 419
How to tell acid from base?
Hi, I have a general question about acids and bases. If you are given a chemical equation, how can you tell which molecule is an acid or base, electron acceptor or electron receiver?
- Sat Jun 02, 2018 4:22 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Problem 4.27
- Replies: 4
- Views: 544
Problem 4.27
Hi,
My question is why is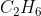 non polar. Since it is tetrahedral, wouldn't the dipole moments not cancel out?
non polar. Since it is tetrahedral, wouldn't the dipole moments not cancel out?
My question is why is
- Tue May 29, 2018 8:12 pm
- Forum: Lewis Structures
- Topic: Central atom and octet rule?
- Replies: 12
- Views: 3077
Re: Central atom and octet rule?
Can elements such as iodine exceed the octet rule? Can it form double and triple bonds even though it has 7 valence e-?
- Tue May 29, 2018 6:08 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Homework 4.7
- Replies: 3
- Views: 460
Homework 4.7
Problem seven asks for how many different bond angles there are for OSCl. Isn’t there only one value for that angle? Also, it asks for the bond angles of the OSCl and ClSCl? Wouldn’t they be the same?
- Tue May 29, 2018 12:58 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: General Molecular Shape
- Replies: 3
- Views: 385
General Molecular Shape
Hi,
How do tell what the molecular shape of the molecule is? What is the criteria for distinguishing it?
How do tell what the molecular shape of the molecule is? What is the criteria for distinguishing it?
- Tue May 29, 2018 12:56 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 4.3 Homework
- Replies: 3
- Views: 707
4.3 Homework
Hi Everyone,
I am confused on problem 4.3. How can part a) HCN be linear. Wouldn't the lone pair on N cause it to not be linear?
Also, how do you know that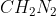 is tetrahedral rather than trigonal planar?
is tetrahedral rather than trigonal planar?
I am confused on problem 4.3. How can part a) HCN be linear. Wouldn't the lone pair on N cause it to not be linear?
Also, how do you know that
- Sat May 26, 2018 8:10 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Types of Bonds
- Replies: 6
- Views: 849
Types of Bonds
Hi Everyone,
I am a bit confused on the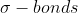 and the
and the 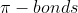 . I get that they are two different types of bonds and that they occur when to elements bond, but I don't quite understand what the difference is between the two? What makes them different?
. I get that they are two different types of bonds and that they occur when to elements bond, but I don't quite understand what the difference is between the two? What makes them different?
I am a bit confused on the
- Sat May 26, 2018 5:21 pm
- Forum: Ionic & Covalent Bonds
- Topic: Group 17
- Replies: 2
- Views: 452
Re: Group 17
Yes, in some cases, group 17 elements can form double or triple bonds because some of them can have an expanded octet depending on where they are on the periodic table. For example, I believe Iodine can form a double bond because it is able to have an expanded octet.
Hope this helps!
Hope this helps!
- Sat May 26, 2018 5:14 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Polar Bonds versus non polar bonds
- Replies: 2
- Views: 361
Re: Polar Bonds versus non polar bonds
To determine whether a molecule is polar or non-polar, you look at the dipole moments. If there are no dipole moments or the dipole moments cancel each other out, then the molecule is non-polar. If there are dipole moments that don't cancel out, then the molecule is polar. CCl_{4} is non-polar becau...
- Fri May 18, 2018 9:20 am
- Forum: Resonance Structures
- Topic: 3.47
- Replies: 3
- Views: 612
Re: 3.47
I believe that if a problem is drawn a certain way (square or chain), they will tell us as they did in 47 and 43. If we haven't learned that, or we are not expected to know it, then they usually tell us what information we need to complete the problem.
- Fri May 18, 2018 9:16 am
- Forum: Resonance Structures
- Topic: HW problem 3.45
- Replies: 4
- Views: 585
Re: HW problem 3.45
N only has 5 valence electrons, and once you single bond to both O's and the Cl, you only have 1 lp (2 e-s) left. That being said, it can only form one more bond, and once it does, you can count all the bonds Nitrogen makes (4, each representing 2 e-s) which will equal 8 e-s (Octet). If it formed 2 ...
- Fri May 18, 2018 9:06 am
- Forum: Formal Charge and Oxidation Numbers
- Topic: 3.53
- Replies: 4
- Views: 829
Re: 3.53
Yes, If I am not mistaken that is what the problem is asking. The closest one to 0 is more stable, so it is less likely to be very reactive.
- Mon May 07, 2018 10:06 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Quantum Mechanics Worksheet #9 [ENDORSED]
- Replies: 4
- Views: 926
Re: Quantum Mechanics Worksheet #9 [ENDORSED]
B would be impossible because if n=3, then l can only go to 2 because the l maximum is (n-1). So at n=3 energy level, you can have 3 subshells (l) which are 0,1, and 2. Keep in mind that l=0 is s, l=1 is p, and l=2 is d. D would be impossible for the same reasoning. When n=3, l can only go up to n-1...
- Mon May 07, 2018 9:59 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Midterm Question
- Replies: 2
- Views: 396
Re: Midterm Question
Yes, I was told that that they will mark off for the wrong amount of sig figs.
- Mon May 07, 2018 8:03 pm
- Forum: Lewis Structures
- Topic: Ammonium Sulfate Example in Notes
- Replies: 2
- Views: 362
Ammonium Sulfate Example in Notes
Hi, I am having trouble understanding how the professor figured out how many electrons are in (NH_{4})_{2}SO_{4} . It says that there are 5 electrons in N and 6 electrons in O. How do you know how many are in the valence shell? I thought for N it would have 3 valence e- because it is third i...
- Mon May 07, 2018 1:32 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: homework 2.43 part e [ENDORSED]
- Replies: 8
- Views: 1001
Re: homework 2.43 part e [ENDORSED]
But why is it 5d^4 6s^2 and not 5d^5 6s^1? I thought it was more stable to have d^5 than d^4 (as well as d^10 instead of d^9)? Also are we expected to know how to include the f-block in electron configurations? I'm not sure but I thought we had to know how to do electron configurations of the s-blo...
- Sat May 05, 2018 1:38 pm
- Forum: Balancing Chemical Reactions
- Topic: Test 1 Q3
- Replies: 2
- Views: 454
Test 1 Q3
Hi, I was wondering if someone could help me balance and equation. The hard part for me to understand is when you have to use a fraction and the multiply to get whole numbers. The equation is below:
_{2} + NO + H_{2}O)
- Thu May 03, 2018 3:27 pm
- Forum: Ionic & Covalent Bonds
- Topic: Octet
- Replies: 7
- Views: 1015
Octet
On Wednesday the professor briefly talked about octets, when adding or removing an electron, and I am really sorry if this is a dumb question, but what exactly does he mean by an octet? It is something I don't remember from high school chemistry.
- Thu May 03, 2018 1:48 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: f orbital
- Replies: 1
- Views: 220
f orbital
Hi Everyone. I have a question about the f orbital. Since the f orbital begins at 57 (Lantharium), how would you write the electron configuration for that? Would it come after 6s? And if so, then what would 71 (Lutetium) be? Sorry if this question is confusing, I just want to make sure I understand ...
- Sat Apr 28, 2018 12:07 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: orbital values [ENDORSED]
- Replies: 2
- Views: 3051
orbital values [ENDORSED]
I have some questions about orbital values.
1. What exactly is n?
2. If l can be 0,1,...,n-1, then how can you figure out what value of l it is?
3. If there is different options of what m can be, then how do you figure out what value of m it actually is?
4. What exactly does l and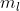 mean?
mean?
1. What exactly is n?
2. If l can be 0,1,...,n-1, then how can you figure out what value of l it is?
3. If there is different options of what m can be, then how do you figure out what value of m it actually is?
4. What exactly does l and
- Fri Apr 27, 2018 7:39 pm
- Forum: *Shrodinger Equation
- Topic: Schrodinger Equation [ENDORSED]
- Replies: 8
- Views: 934
Re: Schrodinger Equation [ENDORSED]
What does the 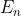 equation mean in this instance. I guess I am confused on what we use the Schrodinger Equation for?
equation mean in this instance. I guess I am confused on what we use the Schrodinger Equation for?
- Tue Apr 24, 2018 6:08 pm
- Forum: DeBroglie Equation
- Topic: HW 1.33
- Replies: 12
- Views: 1272
Re: HW 1.33
Hi! The information given to us in this question is that no electron is emitted until the frequency of radiation reaches 2.50*10^16 Hz. We are trying to find the amount of energy required to remove the electron from the surface, thus the threshold energy (also known as work function) required to re...
- Sat Apr 21, 2018 4:49 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Heisenberg Uncertainty Question [ENDORSED]
- Replies: 2
- Views: 252
Heisenberg Uncertainty Question [ENDORSED]
Hi Everyone,
My question is "What is the numerical cutoff for whether the value you get for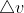 or
or 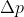 is possible/helpful or not?"
is possible/helpful or not?"
My question is "What is the numerical cutoff for whether the value you get for
- Mon Apr 16, 2018 6:05 pm
- Forum: Properties of Light
- Topic: HW 1.15
- Replies: 3
- Views: 472
HW 1.15
Hi Everyone, I am working on the homework and am trying yo figure out problem 1.15 but I am having a little trouble getting started. I know that it gave us the wavelength, and I am assuming we are supposed to use Rydberg's equation for it, but other than that, I am not quite sure what to do. I just ...
- Mon Apr 16, 2018 1:43 pm
- Forum: Properties of Light
- Topic: Sig Figs
- Replies: 2
- Views: 247
Sig Figs
Hi, I have a quick question about significant figures. If I have an answer that comes out to be 600nm, but I want to only have 2 sig figs in my answer, how would I write that? Could I write 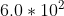 ?
?
- Sat Apr 14, 2018 2:35 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Heisenberg Uncertainty Concept Question [ENDORSED]
- Replies: 1
- Views: 358
Heisenberg Uncertainty Concept Question [ENDORSED]
Hi, Would someone be able to explain the concept of this question? I am just confused on how the precision relates to each other.
- Sat Apr 14, 2018 2:29 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Heisenberg Uncertainty, Post-Module #17
- Replies: 1
- Views: 110
Heisenberg Uncertainty, Post-Module #17
Hi, I have a question on number 17 of the post-module question. Since we know that the uncertainty I speed is 1/100 speed of light, so 3.0*10^{6} , so doubled 6.0*10^{^{6}} , how would we go about solving the rest of the equation? would you start by plugging that into \Delta p=m*(6.0*10^{6})...
- Mon Apr 09, 2018 8:09 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: L.3
- Replies: 1
- Views: 297
L.3
Hi Everyone! I am trying to do problem L.3 and am a bit confused. I tried doing it as the the book shows in the tool box in the book and I get all the way to the part where you convert Li3N into H2: (\frac{.0015}{34.83})mol Li_{3}N * \frac{2 mol H_{2}}{1 molLi_{3}N} The solutions manual had ...
- Sat Apr 07, 2018 2:48 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: G.5 Part A) Molarity [ENDORSED]
- Replies: 9
- Views: 978
Re: G.5 Part A) Molarity [ENDORSED]
I also don't understand this problem. I tried looking at the solution guide to see how it went about solving it, but I am a bit confused on what the steps are that the solution is doing. Would someone be able to explain the steps to this problem?
- Sat Apr 07, 2018 2:28 pm
- Forum: Balancing Chemical Reactions
- Topic: Subscripts [ENDORSED]
- Replies: 6
- Views: 917
Re: Subscripts [ENDORSED]
The 2 outside the parentheses can be multiplied by the three inside which tells you that you have 6 Nitrogens right there. That being said, you now just need to make sure that the other side has the same amount, which you can do by balancing the rest!
- Sat Apr 07, 2018 2:19 pm
- Forum: Limiting Reactant Calculations
- Topic: In-Class Limiting Reactant and Theoretical Yield Problem [ENDORSED]
- Replies: 3
- Views: 367
In-Class Limiting Reactant and Theoretical Yield Problem [ENDORSED]
Hi Everyone, I have a question on the last problem that the professor did on Friday in week one. The problem was: Solid Calcium Carbide CaC2 reacts with H2O to for aqueous Calcium Hydroxide, Ca(OH)2 and Ethyne gas C2H2. What is the limiting reactant when 100g H2O reacts with 100g CaC2? What is the t...

