Which should we use on final:
Sum of bonds broken - sum of bond formed
Or
Sum of bonds in reactant side - sum of bonds in product side
Search found 60 matches
- Sat Mar 16, 2019 10:28 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bond enthalpy
- Replies: 2
- Views: 457
- Sat Mar 16, 2019 10:25 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Drawing cells
- Replies: 3
- Views: 649
Drawing cells
What do we need to include/label in drawing a concentration cell?
When I google a picture of a cell there’s so much information
When I google a picture of a cell there’s so much information
- Sat Mar 16, 2019 10:17 pm
- Forum: Balancing Redox Reactions
- Topic: Acidic conditions
- Replies: 3
- Views: 547
Acidic conditions
How is balancing any different if there are acidic conditions (asking on question 3b in Lyndon’s Pork Ramen review)
- Wed Mar 13, 2019 12:00 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell diagram
- Replies: 3
- Views: 424
Re: Cell diagram
What would be an example of a conductive metal? Should we have some memorized?
- Sun Mar 10, 2019 10:09 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Arrhenius plot
- Replies: 4
- Views: 449
Re: Arrhenius plot
I attached a graph from Wikipedia if that helps!
- Sun Mar 10, 2019 10:07 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Arrhenius plot
- Replies: 4
- Views: 449
Re: Arrhenius plot
I believe it’s because it displays a straight line so it’s easier to say more things about its slope since it’s a straight line.
If it was all wiggly I imagine it might be harder to make estimates or assume what K is for example.
If it was all wiggly I imagine it might be harder to make estimates or assume what K is for example.
- Sun Mar 10, 2019 10:03 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Activation Energy
- Replies: 16
- Views: 1509
Re: Activation Energy
Activation energy is the energy needed for a reaction to happen (for reactants to turn into products or vice versa) You can show this using graphs by drawing a little hump (if you google activation energy exothermic / endothermic they’ll have all the graphs showing this) If the hump goes from higher...
- Sun Mar 10, 2019 9:56 pm
- Forum: First Order Reactions
- Topic: half life
- Replies: 2
- Views: 340
Re: half life
You should use half life if there’s a questions asking how long does it take for the concentration of “A” to get to a certain percentage of its original concentration
Or when a questions asks how much of A concentration is left after certain years if it’s half life is 10 years or so, for example.
Or when a questions asks how much of A concentration is left after certain years if it’s half life is 10 years or so, for example.
- Sat Mar 02, 2019 9:16 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Ion selective electrode
- Replies: 1
- Views: 182
Ion selective electrode
What does it mean for an electrode to be ion selective?
What’s the difference between an electrode and an electron?
What role do ion selective electrons play in electrolysis?
What’s the difference between an electrode and an electron?
What role do ion selective electrons play in electrolysis?
- Sat Mar 02, 2019 9:10 pm
- Forum: General Rate Laws
- Topic: Unique vs instantaneous rate
- Replies: 6
- Views: 632
Unique vs instantaneous rate
In Friday’s lecture, professor Lavelle explain the difference between the unique rate and the instantaneous rate but I don’t think I understood
Could anyone explain?
Could anyone explain?
- Sat Mar 02, 2019 9:09 pm
- Forum: General Rate Laws
- Topic: 1st order reaction
- Replies: 1
- Views: 176
1st order reaction
What does it mean for a reaction to be first or second order? Did we cover this in 14a or 14b?
- Sun Feb 24, 2019 8:31 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell diagram
- Replies: 3
- Views: 344
Re: Cell diagram
Also the professor also showed us the cell diagram in writing form and omgg I feel like there is so much going on. Could someone explain it please ?
- Sun Feb 24, 2019 8:30 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Spontaneous?
- Replies: 13
- Views: 1588
Spontaneous?
How do you know if delta S, H, or G are spontaneous?
- Sun Feb 24, 2019 8:29 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Meaning of E
- Replies: 3
- Views: 1189
Meaning of E
What is the difference between E and E°?
- Sun Feb 24, 2019 8:28 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell diagram
- Replies: 3
- Views: 344
Cell diagram
in lecture, the professor showed a cell diagram with a little line labeled circuit
What is this? Like a copper wire that transmits electrons ?
What is this? Like a copper wire that transmits electrons ?
- Sun Feb 17, 2019 5:47 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Activation energy
- Replies: 8
- Views: 866
Activation energy
What is the relationship between activation energy and kinetic control?
- Sun Feb 17, 2019 5:44 pm
- Forum: Calculating Work of Expansion
- Topic: Work
- Replies: 8
- Views: 952
Work
There are so many work equations and equations derived from others
How do you know which to use? Do you need to memorize each or know how to derive them?
Thanks in advance
How do you know which to use? Do you need to memorize each or know how to derive them?
Thanks in advance
- Sun Feb 17, 2019 5:42 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Enthalpy and graphs
- Replies: 2
- Views: 308
Enthalpy and graphs
What is the relationship between enthpy and exothermic/endothermic reactions?
Can any explain the two graphs we saw in class ? (One has a flat line and the other had a curved line)
Can any explain the two graphs we saw in class ? (One has a flat line and the other had a curved line)
- Sun Feb 10, 2019 11:13 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Work
- Replies: 2
- Views: 237
Work
How do you know if work was done on the system or if system did work on its surroundings? I know it’s a difference of work being negative but I don’t understand why
- Sun Feb 10, 2019 11:10 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: q=mCdeltaT
- Replies: 15
- Views: 8195
q=mCdeltaT
I’ve seen this equation written as q=nCdeltaT and q=mCdeltaT
Should we use moles or grams for this equation on the test?
Should we use moles or grams for this equation on the test?
- Sun Feb 10, 2019 11:06 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Entropy at quilibrium
- Replies: 2
- Views: 335
Entropy at quilibrium
Why is entropy at its maximum when it’s at equilibrium ?
- Mon Feb 04, 2019 9:30 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 8.19
- Replies: 3
- Views: 720
8.19
Hi I have no idea how to approach this problem: Calculate the heat that must be supplied to a 500g Cu kettle containing 400g of water to raise its temperature from 22°C to 100°C I know heat is q but I can’t seem to find an equation with q and temperature in the lecture notes. Any help is appreciated!
- Sun Feb 03, 2019 11:07 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Types of calorimetry
- Replies: 1
- Views: 260
Types of calorimetry
What is the difference between constant P calorimetry and constant V calorimetry? How do they work? When would we use them?
- Sun Feb 03, 2019 11:04 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Types of enthalpy
- Replies: 2
- Views: 405
Types of enthalpy
What is the difference between  and standard enthalpy of formation
and standard enthalpy of formation 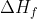 ° ?
° ?
How do we know which to use?
How do we know which to use?
- Sun Feb 03, 2019 10:57 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Internal energy
- Replies: 5
- Views: 563
Internal energy
What is internal energy and how do we find it?
- Fri Jan 25, 2019 9:11 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Method 2
- Replies: 3
- Views: 381
Method 2
Under method two- additional comments from the notes it says “ 2) bond enthalpies of diatomic molecules are accurate (measured for those molecules)” what does this mean? And enthalpies of diatomic molecules are more accurate as opposed to what, exactly?
Thank you!
Thank you!
- Fri Jan 25, 2019 9:08 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: adding two reactions together
- Replies: 2
- Views: 274
adding two reactions together
Can anyone show me how to add to reactions together for Hess’s law? Or give an example?
Thank you!
Thank you!
- Fri Jan 25, 2019 9:07 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: State properties
- Replies: 3
- Views: 360
State properties
In Wednesday’s lecture professor Lavelle said enthalpy has state properties but heat does not
Can anyone explain to me
Thank you!
Can anyone explain to me
Thank you!
- Sun Jan 20, 2019 6:24 pm
- Forum: Conjugate Acids & Bases
- Topic: Writing equations
- Replies: 3
- Views: 715
Writing equations
I took 14A a long time ago so I’m really rusty when it comes to writing equations Can someone help me by explaining the steps to writing an equation where a weak base/acid reacts with water to make H3O or OH? Like how do you know where the H/proton is being transferred to? (For example in NH3 + H2O ...
- Sun Jan 20, 2019 6:19 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ICE box
- Replies: 1
- Views: 186
ICE box
Can someone tell me when we would use the ice box, what are the steps to it and also
when is it better to assume x is a really small number instead of just using the quadratic formula? (Like 1.01-x becoming just 1.01 if we assume x is really tiny)
when is it better to assume x is a really small number instead of just using the quadratic formula? (Like 1.01-x becoming just 1.01 if we assume x is really tiny)
- Sun Jan 20, 2019 12:31 am
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: pKa and Ka
- Replies: 1
- Views: 539
pKa and Ka
In problem 12.35 (6th Ed) they ask us to get Ka from pKa which is fine you just use 10^pKa. However I noticed that the pKa values have numbers right next to them like pKa1=... or pKa2=.... what do they mean by saying pKa2 ? How do you get Ka from that ? (Part d says: give the Ka value for the acid H...
- Sun Jan 13, 2019 7:45 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K vs Q
- Replies: 4
- Views: 401
K vs Q
What are some of the main differences between K and Q? how are they similar?
- Sun Jan 13, 2019 7:44 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Question 11.11
- Replies: 1
- Views: 153
Question 11.11
I don’t understand how the ratio between O2 and O3 is different. How would you predict it just from seeing a equation. Is it because of the molar ratios?
- Sun Jan 13, 2019 7:41 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium constant
- Replies: 6
- Views: 547
Equilibrium constant
How come pressure does not affect Kc? It does affect Kp right?
- Mon Jan 07, 2019 9:24 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Lecture 1
- Replies: 1
- Views: 199
Lecture 1
Hello,
I was not able to go to today’s lecture for chem 14B :( could anyone please let me know what happened today? I will appreciate you and thank you for the rest of the quarter!
I was not able to go to today’s lecture for chem 14B :( could anyone please let me know what happened today? I will appreciate you and thank you for the rest of the quarter!
- Sat Jun 09, 2018 9:09 pm
- Forum: Lewis Acids & Bases
- Topic: Lewis vs Bronsted
- Replies: 4
- Views: 580
Lewis vs Bronsted
hello, can someone explain to me the difference between lewis acids/bases vs bronsteds? Also, what are some key differences between acids and bases? what is important to remember?
- Sat Jun 09, 2018 9:06 pm
- Forum: Hybridization
- Topic: Hibridization
- Replies: 3
- Views: 630
Hibridization
Can someone explain to me how to determine hybridization? What are some key things to remember?
- Sat Jun 09, 2018 9:04 pm
- Forum: Hybridization
- Topic: sigma vs pi bonds
- Replies: 4
- Views: 849
sigma vs pi bonds
I get that a single bond has one sigma bond and that a double bond has one sigma and one pi bond but what is the difference between a sigma bond and a pi bond?
- Sat Jun 09, 2018 8:57 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR
- Replies: 7
- Views: 982
Re: VSEPR
thanks guys helped a lot!
- Sun Jun 03, 2018 10:26 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR
- Replies: 7
- Views: 982
VSEPR
can someone go over important things in determining VSEPR shape? thank you!
- Sun Jun 03, 2018 10:11 pm
- Forum: Sigma & Pi Bonds
- Topic: sigma and Pi bonds
- Replies: 2
- Views: 473
Re: sigma and Pi bonds
i found this picture
hope it helps!
hope it helps!
- Sun Jun 03, 2018 10:06 pm
- Forum: Ionic & Covalent Bonds
- Topic: Polarizability
- Replies: 7
- Views: 904
Re: Polarizability
doesn't this refer to the shape of the electrons like the way they move or something like that? I am also confused on this
- Sun May 27, 2018 1:57 pm
- Forum: Lewis Structures
- Topic: 2 lone pairs
- Replies: 5
- Views: 586
Re: 2 lone pairs
I think it’s important to have symmetry when drawing structures
Hope this helps
Hope this helps
- Sun May 27, 2018 1:54 pm
- Forum: Lewis Structures
- Topic: Single electron
- Replies: 5
- Views: 505
Re: Single electron
Do you mean like a radical?
- Sun May 27, 2018 1:53 pm
- Forum: Bond Lengths & Energies
- Topic: Bond Strength
- Replies: 5
- Views: 831
Re: Bond Strength
(Is this is about that one question in test 3 ?) I went to a UA and asked and he basically said the slight negative charge of the Two molecules made the nucleus of the atom slightly more positive which would have more of an attractive force pulling in other atoms So maybe this makes the double bond ...
- Thu May 17, 2018 1:34 am
- Forum: Ionic & Covalent Bonds
- Topic: Ionic bonds
- Replies: 6
- Views: 873
Ionic bonds
How do we show ionic bonds?
In a covalent bond we can draw a line that shows two atoms sharing electrons
but ionic bonds don't share electrons
So like let's say we're drawing the lewis structure for NaCl
Do we just write Na+ Cl- and that shows a bond?
In a covalent bond we can draw a line that shows two atoms sharing electrons
but ionic bonds don't share electrons
So like let's say we're drawing the lewis structure for NaCl
Do we just write Na+ Cl- and that shows a bond?
- Thu May 17, 2018 1:15 am
- Forum: Lewis Structures
- Topic: Central atom
- Replies: 7
- Views: 884
Re: Central atom
I agree with Joanna,
The central atom should I always be the one with the least electronegativity
The trend in periodic table increases going up and to the right
Out of Cl, O, and C in this example C is the most left so it has the least electronegativity
The central atom should I always be the one with the least electronegativity
The trend in periodic table increases going up and to the right
Out of Cl, O, and C in this example C is the most left so it has the least electronegativity
- Thu May 17, 2018 1:10 am
- Forum: Lewis Structures
- Topic: HW 3.41
- Replies: 2
- Views: 358
Re: HW 3.41
I think it's right ( it might just be a matter of structure but I don't think we've learned about that yet)
Also, would this be resonance?
Also, would this be resonance?
- Fri May 11, 2018 11:44 pm
- Forum: Trends in The Periodic Table
- Topic: electron affinity [ENDORSED]
- Replies: 15
- Views: 2023
Re: electron affinity [ENDORSED]
The way a UA described it to me was that electronegativity is the ability of a neutral atom to attract electrons towards itself and electron affinity is the energy released when the electron is added to the neutral atom (kind of like a cause and effect I think) hope this helps
- Fri May 11, 2018 11:30 pm
- Forum: Lewis Structures
- Topic: NO
- Replies: 3
- Views: 188
Re: NO
I believe taking a lone pair of electrons from oxygen to make a double bond between N and O is possible and it would make the formal charges of O and N zero (without the double bond the formal charge of N is +1 and FC of O is -1) So I think creating a double bond would be the way to go \dddot{N}= {\...
- Fri May 11, 2018 11:15 pm
- Forum: Octet Exceptions
- Topic: expanded octet XeF4
- Replies: 8
- Views: 2199
Re: expanded octet XeF4
it's because F it does not follow the rules to have an expanding octet meaning that it is not possible for F to take anymore electrons since it is in the second row (elements from third row and down can get expanding octets from d orbital)
- Tue May 01, 2018 6:26 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Review Sessions [ENDORSED]
- Replies: 3
- Views: 406
Re: Review Sessions [ENDORSED]
A guy went by yesterday in lecture saying there would be a review session Friday from 12 to 1:50 ( I don’t know if he’s from the step up/workshops) I’m not sure if this helps
- Tue May 01, 2018 6:14 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: The order for s,p,d
- Replies: 3
- Views: 587
Re: The order for s,p,d
In my step up section someone said that the order is dsp as well (ex: 3d10 4s2 4p6). However they also said that if the d orbitals are not full then the order is actually sdp (ex: 4s2 3d5 instead of 3d5 4s2).
Is that right? If so doesn’t that go against the whole writing from least to highest energy?
Is that right? If so doesn’t that go against the whole writing from least to highest energy?
- Tue May 01, 2018 6:07 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Cr and Cu exceptions
- Replies: 3
- Views: 529
Cr and Cu exceptions
I understand that Cu and Cr are exceptions when it comes to electron configurations since the d orbital is not full so then electrons in the s orbital go down a level to have a balanced amount of electrons in d. Does this mean that the elements in the same group will behave similarly? Also, is it po...
- Sun Apr 22, 2018 8:16 pm
- Forum: *Shrodinger Equation
- Topic: ch.2.1
- Replies: 5
- Views: 557
Re: ch.2.1
I think what it means is that e- are pulled by the nucleus and therefore can't escape I suppose? Like a particle that is stuck inside a box.
- Sun Apr 22, 2018 8:13 pm
- Forum: Properties of Light
- Topic: Homework 1.5
- Replies: 6
- Views: 634
Re: Homework 1.5
gamma rays have a higher energy since they have a higher frequency and a shorter wavelength (it helps to look at pictures of this)
- Sun Apr 22, 2018 8:05 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Rydberg Formula
- Replies: 4
- Views: 438
Re: Rydberg Formula
I asked my TA about which equation to use and he said that we can derive the same answer from either or so we can use whichever we want
- Sun Apr 08, 2018 10:02 pm
- Forum: General Science Questions
- Topic: Naming compunds
- Replies: 3
- Views: 578
Re: Naming compunds
section in the textbook? or here on the website? I also have this question
- Sun Apr 08, 2018 9:54 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Electron Configuration
- Replies: 1
- Views: 256
Re: Electron Configuration
I think it's something that we will review later on since hybridization is mentioned in outline 4 "Molecular Shape and Structure"
- Sun Apr 08, 2018 9:48 pm
- Forum: Significant Figures
- Topic: Why are sig figs important?
- Replies: 13
- Views: 16218
Re: Why are sig figs important?
I believe we use them to be as exact as possible. For example, when measuring grams of a compound, one might get different results depending in how they round (sig figs are important in rounding). Other than using them in actual lab, I think we use them so we know how to deal with decimals (there ca...

