for the product will be
Search found 89 matches
- Sun Mar 17, 2019 6:20 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Rate of consumption
- Replies: 3
- Views: 639
Re: Rate of consumption
Yes for rate of consumption it is going to be 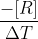 where as rate of formation
where as rate of formation
for the product will be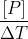 .
.
for the product will be
- Sun Mar 17, 2019 6:17 pm
- Forum: General Rate Laws
- Topic: slow step
- Replies: 11
- Views: 1423
Re: slow step
If you know that the reaction is a pre-equilibrium reaction, then the second step will be the slow one. That first step is going to be a fast reaction and it is considered to be at equilibrium where there is a bottle necking effect and that leads into your second step which is going to be the rate d...
- Sun Mar 17, 2019 6:15 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Bimolecular
- Replies: 13
- Views: 2259
Re: Bimolecular
Bi molecular is a description of how many molecules are participating on the reactants side of an equation. In every case, you would look at the reaction before intermediates or catalysts are removed. If there is only one molecule reacting, then you know it is unimolecular. If you see that there are...
- Sun Mar 10, 2019 3:31 pm
- Forum: First Order Reactions
- Topic: Fractional Rate Constants
- Replies: 2
- Views: 375
Re: Fractional Rate Constants
In this class, you will need to round up, but they do exist as decimals. For the purpose of the class and the exam, just round everything up or down accordingly.
- Tue Mar 05, 2019 2:16 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Textbook Problem 6O #1
- Replies: 1
- Views: 259
Textbook Problem 6O #1
Why do we have to use 2H2O-->O2 + 4H + 4e- in this problem. Also where do they get the value of 1.23 for Enaught and why is Ni2+ half reaction the cathode and not the anode even though we have typically tried to create the most positive E.
- Tue Mar 05, 2019 2:12 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Textbook Problem 6O #3
- Replies: 1
- Views: 251
Textbook Problem 6O #3
Can someone explain to me the reasoning behind this problem? I do not understand what specifically causes water to be reduced over metal and vise versa.
- Sun Mar 03, 2019 5:21 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Corrosion and Ion Selective Electrodes
- Replies: 4
- Views: 521
Re: Corrosion and Ion Selective Electrodes
What did he explicitly talk about for the inert electrodes other than their existence.
- Sun Mar 03, 2019 5:19 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Setting up redox equation for Nernst
- Replies: 2
- Views: 361
Re: Setting up redox equation for Nernst
Are we not supposed to use the Appendix for such a question?
- Sun Mar 03, 2019 5:18 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Large K value
- Replies: 5
- Views: 587
Re: Large K value
If Ecell is negative, does that meant that the reverse reaction is favorable since the forward is not?
- Sun Feb 24, 2019 6:01 pm
- Forum: Balancing Redox Reactions
- Topic: Test 2 Material
- Replies: 13
- Views: 1361
Re: Test 2 Material
yes, you do need to know how to do that, as well as how to balance in an acidic solution. That will likely be one of the questions on the exam.
- Sun Feb 24, 2019 5:59 pm
- Forum: Balancing Redox Reactions
- Topic: TEST
- Replies: 7
- Views: 868
Re: TEST
I believe it is all of Gibbs, which includes some of the thermo and everything until nest, but not including it.
- Sun Feb 24, 2019 5:58 pm
- Forum: Balancing Redox Reactions
- Topic: Order to balance
- Replies: 6
- Views: 577
Re: Order to balance
When balancing reactions, you:
1. Balance the elements
2. balance the O
3. Balance the H
4. Balance the electrons
1. Balance the elements
2. balance the O
3. Balance the H
4. Balance the electrons
- Sun Feb 17, 2019 4:23 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Differences in Gibbs Free Energy
- Replies: 3
- Views: 422
Re: Differences in Gibbs Free Energy
In a general sense, why are we looking at the K value when solving problems with Gibbs free energy?
- Sun Feb 17, 2019 4:21 pm
- Forum: Van't Hoff Equation
- Topic: Concept behind Van't Hoff Equation
- Replies: 5
- Views: 701
Re: Concept behind Van't Hoff Equation
In what situations would we need to calculate different temperatures?
- Sun Feb 17, 2019 4:20 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy Definition
- Replies: 4
- Views: 442
Re: Gibbs Free Energy Definition
What exactly is Gibbs free energy, and how does it related to entropy and enthalpy without strictly looking at the equations.
- Sun Feb 10, 2019 4:50 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Negative q
- Replies: 7
- Views: 2773
Re: Negative q
It is when energy is released from a system to an environment, meaning the reaction is going to be exothermic. Depending on the problem, it may make you calculate q, and that just means energy is released.
- Sun Feb 10, 2019 4:48 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: why is H negative when calculating S?
- Replies: 4
- Views: 456
Re: why is H negative when calculating S?
It is because fusion is the opposite of freezing.
- Sun Feb 10, 2019 4:46 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Thermal Disorder
- Replies: 1
- Views: 280
Re: Thermal Disorder
There are few general problems in the text book which have you analyze systems and determine which express more disorder. I believe it is section 4H.
- Sun Feb 10, 2019 4:44 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Constant Pressue
- Replies: 3
- Views: 527
Re: Constant Pressue
Most of the time it will given an indicator that pressure has not changed, but in the case that it doesn't, I think it is safe to assume that there is not pressure change. With that being said, just read the problem carefully.
- Sun Feb 10, 2019 4:43 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Residual Entropy
- Replies: 5
- Views: 491
Re: Residual Entropy
Do more orientations mean a greater residual entropy?
- Sun Feb 03, 2019 7:32 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: delta H vs q
- Replies: 9
- Views: 921
Re: delta H vs q
When pressure is constant, q becomes qp and this is equal to 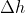 .
.
- Sun Feb 03, 2019 7:28 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard Enthalpy Formation of an element
- Replies: 4
- Views: 462
Re: Standard Enthalpy Formation of an element
Does the standard enthalpy of 0 only apply to diatomic molecules like H, O, F, Cl, Br, I, N?
- Sun Feb 03, 2019 7:26 pm
- Forum: Phase Changes & Related Calculations
- Topic: Compression Work
- Replies: 3
- Views: 369
Re: Compression Work
Work is being done on they system by compressing it. Following the equation work= Pressure*area*distance, it will give you a positive value.
- Sat Jan 26, 2019 10:50 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bonds being broken in reaction
- Replies: 4
- Views: 372
Re: Bonds being broken in reaction
It is both conceptual and applicable. There were two ways to look at it, the specific direct bonds that were broken and subtract the energy needed to form new specific bonds from it. Or you can look at all the energies that form bonds in a molecule and subtract all the energies that form bonds in th...
- Sat Jan 26, 2019 10:41 pm
- Forum: Phase Changes & Related Calculations
- Topic: Phase Changes and Temperature
- Replies: 3
- Views: 373
Re: Phase Changes and Temperature
The reason that temperature stays the same is that, energy required to break bonds is greater than the energy needed to raise the temperature. Since the temperature is already high enough in the process of changing phases, the rest of the energy is put into breaking the bonds of the substance to cha...
- Sat Jan 26, 2019 10:38 pm
- Forum: Phase Changes & Related Calculations
- Topic: Due date for Discussion Questions
- Replies: 8
- Views: 875
Re: Due date for Discussion Questions
They are due Sunday at 11:59 pm
- Sat Jan 26, 2019 10:36 pm
- Forum: Phase Changes & Related Calculations
- Topic: Enthalpy signs
- Replies: 13
- Views: 1715
Re: Enthalpy signs
When deltaH is positive, that mean that the reaction is going to absorb heat in order to proceed and is endothermic. When deltaH is negative, that means that the reaction is going to release heat into the environment and is exothermic.
- Sat Jan 19, 2019 12:45 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Catalysts
- Replies: 7
- Views: 711
Re: Catalysts
Catalysts only speed up the rate of the reaction. They do not have anything to do with the favoring of one side of the reaction or another. They only get you to the products that you want faster and nothing more.
- Sat Jan 19, 2019 12:45 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Pressure
- Replies: 3
- Views: 454
Re: Pressure
-If the pressure increases, the side with less concentration/mols will be favored.
-If the pressure decreases, the side with more concentration/mols will be favored.
-If the pressure decreases, the side with more concentration/mols will be favored.
- Sat Jan 19, 2019 12:43 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: ignoring solids
- Replies: 6
- Views: 765
Re: ignoring solids
You ignore pure liquids because only a small amount ends up reacting, meaning the amount in the products and reactants are practically the same and cancel out when you put the liquid in both the denominator and numerator of the equilibrium ratio. As for solids, there is no such thing as a concentrat...
- Sat Jan 19, 2019 12:41 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Weak Acids and Bases
- Replies: 7
- Views: 828
Re: Weak Acids and Bases
From what I know, if you see that an acid has a really small Ka value, then it will be weak. Same goes for Kb, if it is really small, generally the 10^-3 is a good marker for both, then it will be weaker.
- Sat Jan 19, 2019 12:39 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Example in class
- Replies: 5
- Views: 582
Re: Example in class
Q looks at the system when it is not at equilibrium(when it ratio of products to reactants is equal to Kc). Q is compared to Kc to see whether the reaction proceeds to the right or the left. If Q<Kc then the reaction will "shift" to the right. If Q>K then the reaction will "shift"...
- Sat Jan 12, 2019 10:45 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Solids and Liquids in Rxn
- Replies: 6
- Views: 606
Re: Solids and Liquids in Rxn
If it was solids and liquids, then there would not be an equilibrium constant. There is no such thing as a concentration of a solid. Additionally, for pure liquids, they do not react significantly in the reaction. This means that the amount of pure liquid in the reactants is almost the same as that ...
- Sat Jan 12, 2019 10:41 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Q and K
- Replies: 13
- Views: 1335
Re: Q and K
K is the actual equilibrium constant that is a specific given value for a certain reaction. However, when you are calculating whether a reaction has reached equilibrium, you use Q, and compare it to the equilibrium constant K. If Q is equal to K, then the reaction is at equilibrium. Since K is a fix...
- Sat Jan 12, 2019 10:36 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kc and Kp
- Replies: 12
- Views: 1755
Re: Kc and Kp
The only thing included is aqueous solutions and gases but not liquids or solids. You cannot have a concentration of a liquid and pure liquids do not really react much so that amount that existed in the reactants will almost be the exact same in the products which will just cancel out in the calcula...
- Sat Jan 12, 2019 10:36 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ice table
- Replies: 4
- Views: 449
Re: ice table
Simply put, look at the molar coefficients on each of the molecules and using that number, it will be the value of X in the ICE table. If you are looking at the reactants, you will subtract X. However, if you are looking at products, then you are adding X.
- Mon Jan 07, 2019 9:22 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Solids and Liquids and the K expression
- Replies: 2
- Views: 818
Re: Solids and Liquids and the K expression
Solids and liquids are not included in the K expression because there is no such thing as a concentration of a solids. For liquids, the molar concentration of a pure substance, like water, does not change in a reaction. The amount of liquid that reacts in a reaction is so small that both amounts of ...
- Sun Dec 09, 2018 5:18 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Strength of Acids vs. Number of Oxygens
- Replies: 6
- Views: 1478
Re: Strength of Acids vs. Number of Oxygens
Because there is more ability for resonance to stabilize the anion after the proton is donated. This means that there is a better distribution of charge and makes the acid more strong.
- Sun Dec 09, 2018 5:16 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Ligands
- Replies: 6
- Views: 1039
Re: Ligands
porphyrin is a chelate that binds in 4 places, thus making it tetradentate
- Sun Dec 09, 2018 5:15 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Why HClO2 is a stronger acid than HBrO2?
- Replies: 6
- Views: 12895
Re: Why HClO2 is a stronger acid than HBrO2?
Since there is an equal amount of oxygens, you look at which is more electronegative (Br or Cl) and since Cl is more electronegative, it is the stronger acid.
- Sun Dec 09, 2018 5:13 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Strengths of H2S vs H2Se
- Replies: 4
- Views: 9170
Re: Strengths of H2S vs H2Se
Bigger london dispersion forces do lead to higher boiling points when a molecule exhibits no hydrogen bonds, is not polar or when the polarity is almost the same between two molecules. The bigger the atom, the greater the shift in electrons to create a temporary dipole and makes H2Se a stronger acid
- Sun Dec 09, 2018 5:09 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Memorizing Acids by name
- Replies: 3
- Views: 736
Re: Memorizing Acids by name
Dont memorize but understand. There are 3 general rules that you can look at that the book describes intensively. 1. The more 0, the stronger the acid 2. The more electronegative the molecule, the more acidic. 3. The easier to break up a bond, the more electronegative and that looks at things like H...
- Sun Dec 09, 2018 5:04 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Boiling point
- Replies: 4
- Views: 806
Re: Boiling point
Since the difference in electronegativity between Se and S is negligible, we are considering their dipoles as almost the same. The only thing we can look at now is london forces and since Se is bigger, it is going to create a bigger temporary dipole and have stronger london forces.
- Sun Dec 09, 2018 5:03 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Why HF is a weaker acid than HCl
- Replies: 17
- Views: 16268
Re: Why HF is a weaker acid than HCl
In this case, you are looking at the HF bond length vs the HCL bong length. Since Florine is more elctronegative than Cl, it will have a stronger pull on the H make it harder to donate the H. The longer the bond, the easier it is to remove the H. It follows one of three rules in the book for stronge...
- Sun Dec 02, 2018 11:55 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Polydentate
- Replies: 2
- Views: 316
Re: Polydentate
To know if something is a polydentate ligand, you need to see if it has multiple places where it can bind to through multiple lone electron pairs. The only way I know of finding this out is by drawing out the lewis structure and looking at is electron geometry to determine if it is polydentate or not.
- Sun Dec 02, 2018 11:49 pm
- Forum: Naming
- Topic: Polydentate Ligands
- Replies: 2
- Views: 323
Re: Polydentate Ligands
Polydentate ligands are ligands that have multiple areas where they can bond. However, ligands that only have one binding site, like NH3 would be monodentate.
- Sun Dec 02, 2018 11:44 pm
- Forum: Naming
- Topic: drawing ligands
- Replies: 6
- Views: 562
Re: drawing ligands
He just wanted to illustrate that the central atom can connect to any one of the elements in that particular compound.
Re: Naming
Memorize the ones that you see come up in the problem set. Those are the ones that are usually brought up during the test but if you can memorize anymore, I would definitely do it to play it safe.
- Sun Dec 02, 2018 2:24 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Coordination #
- Replies: 2
- Views: 185
Re: Coordination #
I would think of it as areas that are bonded around the central atom. It is similar to the electron geometry structures of compounds, but rather than looking at area of electron density, you look at areas with atoms and ions.
- Sun Dec 02, 2018 2:22 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: chelate
- Replies: 4
- Views: 475
Re: chelate
A chelate is a made up of many complex compounds where there is a central metal atom that binds to other ligands in a ring/cyclical type structure. I believe the ligand is also polydentrate.
- Sun Dec 02, 2018 2:18 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Coordination Number
- Replies: 10
- Views: 961
Re: Coordination Number
So coordination number corresponds to the areas bonded to the center of a ligand similar to the electron geometry structures?
- Sun Dec 02, 2018 2:16 pm
- Forum: Naming
- Topic: If a ligand has a name with...
- Replies: 5
- Views: 515
Re: If a ligand has a name with...
When something is polydentrate it means that the electron lone pairs are found to be distributed among different atoms on a particular ligand rather than having the electron lone pairs on one atom, where it would not be polydentrate. Hope this helps
Re: Ligands
A ligand is polydentrate when the electron lone pairs are found to be distributed among different atoms on a particular ligand rather than having the electron lone pairs on one atom, where it would not be polydentrate. Hope this helps
- Sun Dec 02, 2018 2:12 pm
- Forum: Naming
- Topic: naming coordination compounds for the final
- Replies: 3
- Views: 408
Re: naming coordination compounds for the final
It means that you must be able to write out the exact order of a compound from left to right. Knowing the general rules for naming them is helpful, but it is more about the application of those rules on the exam. There are many helpful examples provided in the book to give you a better picture of wh...
- Sun Dec 02, 2018 2:09 pm
- Forum: Naming
- Topic: re: Bond Length and Resonance
- Replies: 2
- Views: 340
Re: re: Bond Length and Resonance
Memorize the ones that are provided in the book. Also, we will not be given the names on the exam. You must memorize them.
- Sun Nov 25, 2018 11:55 pm
- Forum: Hybridization
- Topic: Double Bonds
- Replies: 4
- Views: 468
Re: Double Bonds
What do you mean they are found between the p orbitals? Also, what about d orbitals then?
- Sun Nov 25, 2018 11:52 pm
- Forum: Hybridization
- Topic: sigma/pi bonds
- Replies: 7
- Views: 703
Re: sigma/pi bonds
What do you mean by "end to end" or "side to side"?
- Sun Nov 25, 2018 11:46 pm
- Forum: Hybridization
- Topic: hybridization of BeCl2
- Replies: 4
- Views: 374
Re: hybridization of BeCl2
Aside from looking at the regions of electron density in the Lewis dot structure, when you find Be on the periodic table, you see that has 2 valence electrons. When looking at its hybridization, this would give it an s2 denotation. However, we need to have two sites of bonding and through hybridizat...
- Sun Nov 25, 2018 11:42 pm
- Forum: Hybridization
- Topic: Questions in Chapter
- Replies: 5
- Views: 499
Re: Questions in Chapter
They are not going to test us based on our knowledge of a molecule. It will almost always be provided to us on the exam.
- Sun Nov 25, 2018 11:41 pm
- Forum: Hybridization
- Topic: Figuring out the hybirdization
- Replies: 2
- Views: 356
Re: Figuring out the hybirdization
Do you remember the box method that was taught in high school? Each box with one electron inside of it opens itself up to bonding. When looking at the boxes, the s has only one box which allows two total electrons, the p has 3 boxes which allows 6 total electrons, the d has 5 boxes which allow for 1...
- Fri Nov 23, 2018 8:54 pm
- Forum: Hybridization
- Topic: determining shape from given information
- Replies: 3
- Views: 383
Re: determining shape from given information
No because hybridization only tells us how atomic orbitals combine to form hybridized orbitals. These orbitals allow us to determine bonding properties of a molecules which contributes to the overall geometric structure. It is not solely to create the structure of a molecule, mainly because were are...
- Fri Nov 23, 2018 8:45 pm
- Forum: Hybridization
- Topic: when to use hybridization
- Replies: 4
- Views: 416
Re: when to use hybridization
Hybridization helps in generally understanding of how orbitals are made suitable in order to pair electrons so that chemical bonds can form with other atoms. Just know the concept behind it because this topic is just to test your knowledge of how bonds can be made. It will be a supplement to a more ...
- Fri Nov 23, 2018 8:36 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: AXE formula
- Replies: 32
- Views: 12186
Re: AXE formula
The only way for you to know the shapes is to simply memorize them using the table above or one that you can search up online. By knowing AXE, that should in turn help you figure out the different geometric shapes of each molecule.
- Thu Nov 22, 2018 7:58 pm
- Forum: Hybridization
- Topic: Help with Confusion
- Replies: 6
- Views: 598
Re: Help with Confusion
My TA also said that we might be tested on this too, but I think for the test, Dr.Lavelle will not be asking us about it. Regardless, prepare for it anyways in case.
- Thu Nov 22, 2018 7:55 pm
- Forum: Hybridization
- Topic: Determining shape
- Replies: 3
- Views: 326
Re: Determining shape
All though hybridization does relate to VSEPR, its main focus is not about helping you draw the model. All hybridization does is illustrate how many areas of electron density there are in a specific molecule and those fields can either be a boding pair or a lone pair. You can use it to help with sma...
- Thu Nov 22, 2018 7:53 pm
- Forum: Hybridization
- Topic: Hybridization and drawing
- Replies: 3
- Views: 419
Re: Hybridization and drawing
All though hybridization does relate to VSEPR, its main focus is not about helping you draw the model. All hybridization does is illustrate how many areas of electron density there are in a specific molecule and those fields can either be a boding pair or a lone pair. You can use it to help with sma...
Re: Naming
You should look up the general table for all the different structures and memorize or try to picture the bonds themselves online. I find it super helpful too see all of them right next to each other and see how the structures change
- Mon Nov 19, 2018 1:15 am
- Forum: Naming
- Topic: Naming and Polyatomic Ions
- Replies: 6
- Views: 1014
Re: Naming and Polyatomic Ions
We do not need to memorize polyatomic ions as of right now for this course.
- Mon Nov 19, 2018 1:14 am
- Forum: Naming
- Topic: Coordination Compound confusion [ENDORSED]
- Replies: 4
- Views: 714
Re: Coordination Compound confusion [ENDORSED]
Is there a general formula on how to name compounds?
- Mon Nov 19, 2018 1:13 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: ligands?
- Replies: 3
- Views: 674
Re: ligands?
Are there other ligands that we must know too?
- Sun Nov 11, 2018 2:36 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR?
- Replies: 9
- Views: 973
Re: VSEPR?
VSEPR essentially uses the ideas from the Lewis dot structure to model the geometry of molecules using electron pairs that are around the central in order to minimize the repulsion found in between the valence electrons of that specific molecule.
- Sun Nov 11, 2018 2:31 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSPER and bond strength
- Replies: 2
- Views: 162
Re: VSPER and bond strength
From my understanding, VSEPR theory says that repulsion from the lone electron pair exhibits greater repulsion than a bonding pair. Additionally, when there are multiple bonds, they exhibit a greater repulsive force on near by electron areas than do single bonds.
- Sun Nov 11, 2018 2:26 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR determining shape
- Replies: 3
- Views: 350
Re: VSEPR determining shape
To figure out the shape, model out the Lewis dot structure of the molecule, figure out the number of bonding pairs and lone pairs around the main/central atom, use VSEPR to figure out the electron pair shape of the molecule and the VSEPR shape to then figure out the angles between that are found bet...
- Sun Nov 04, 2018 7:03 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Why does PCl5 break the Octet Rule?
- Replies: 8
- Views: 7098
Re: Why does PCl5 break the Octet Rule?
Since P is the least electro-negative, it will be the central element. Considering that anything from group 3 to 7 in the p block has an expanded octet, which includes P, that means that it will break the octet rule.
- Sun Nov 04, 2018 7:00 pm
- Forum: Lewis Structures
- Topic: Expanded Octets
- Replies: 2
- Views: 325
Re: Expanded Octets
Anything from group 3 to 7 in the p block, which are the most common type of used elements, have an expanded octet.
- Sun Nov 04, 2018 6:56 pm
- Forum: Lewis Structures
- Topic: Lewis Structure(s)
- Replies: 3
- Views: 230
Re: Lewis Structure(s)
The exam will specify which formal structure it wants, which can include all 3. I believe that the more 0's there are, the lower the energy of that molecule but just know how to find the charges in general.
- Sat Oct 27, 2018 10:31 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Writing electron configurations [ENDORSED]
- Replies: 5
- Views: 318
Re: Writing electron configurations [ENDORSED]
If you remember the box method for an orbital, you might remember starting with spin up in each individual box before you start putting in spin down but it does not matter really. As long as you are consistent, say you start spin down, do not mix up spins in each box.
- Sat Oct 27, 2018 10:27 pm
- Forum: Trends in The Periodic Table
- Topic: Electron Affinity Trend
- Replies: 9
- Views: 1129
Re: Electron Affinity Trend
The further right you go, the more electro-negative an element is and the further up you go the more electro-negative an element becomes. Elements in the top right corner are the most electro-negative while those in the bottom left are the least electro-negative. Hope that helps..
- Sat Oct 27, 2018 10:18 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Significance of XYZ to PDF orbitals
- Replies: 6
- Views: 562
Re: Significance of XYZ to PDF orbitals
It is basically denoting the orientation. Think of it like a x,y,z graph and imagine the orientation falling on each of the 3 different lines.
- Sun Oct 21, 2018 7:37 pm
- Forum: Properties of Electrons
- Topic: Wavelength Calculations
- Replies: 5
- Views: 641
Re: Wavelength Calculations
Yes all of the constants will be provided on the test. You should go to the website and find the formula sheet and get familiar with it.
- Sun Oct 21, 2018 7:34 pm
- Forum: Properties of Light
- Topic: Test #2
- Replies: 2
- Views: 344
Re: Test #2
Yes the test will be during the discussion.
- Sun Oct 21, 2018 7:34 pm
- Forum: Properties of Light
- Topic: measuring wavelength
- Replies: 5
- Views: 546
Re: measuring wavelength
He said anything that is less than 10^-18 is not detectable. He will make it very obvious and it will be an extreme either direction.
- Sun Oct 14, 2018 3:36 pm
- Forum: Einstein Equation
- Topic: Numbers to memorize [ENDORSED]
- Replies: 37
- Views: 4155
Re: Numbers to memorize [ENDORSED]
Most of the equations will be given on the exam sheet during the exam which you can find on the website if you want to make sure. Rather than memorize them, just make sure you understand each part and know how to manipulate them because that is much more important for exam 2.
- Sun Oct 14, 2018 3:30 pm
- Forum: Properties of Electrons
- Topic: Wave Length Calculations
- Replies: 5
- Views: 405
Re: Wave Length Calculations
It is too small because the amplitude of the wave and its frequency is so minuscule to detect that it almost seems like it does not exist. Using a number multiplied by 10^-15 or 10^-18 is just the smallest we can actually detect and any smaller is just theory.
- Sun Oct 14, 2018 3:26 pm
- Forum: Properties of Light
- Topic: Colors and Frequency
- Replies: 14
- Views: 1661
Re: Colors and Frequency
I believe we had to remember just a general range and not the actual frequencies of each color. Knowing the frequencies of violet, green and red are probably the most that will be needed and everything else will most likely be given.
- Sun Oct 07, 2018 7:41 pm
- Forum: Limiting Reactant Calculations
- Topic: Limiting Reactant
- Replies: 8
- Views: 1298
Re: Limiting Reactant
I think finding the theoretical yield for both reactants that are considered is much easier to get a grasp for what is the limiting reactant but I was curious if there was a faster way that always works.
- Sat Oct 06, 2018 3:33 pm
- Forum: SI Units, Unit Conversions
- Topic: Fundamental E3
- Replies: 6
- Views: 586
Re: Fundamental E3
Dived the number of atoms on each side (meaning 9) by the two respective molar masses and look at the individual weight of each atom for each side. That should help you understand it better on how to balance out each side.
- Sat Oct 06, 2018 3:30 pm
- Forum: SI Units, Unit Conversions
- Topic: Formula Sheet?
- Replies: 6
- Views: 594
Re: Formula Sheet?
If I were you, I would memorize the few that we need for the upcoming test. They aren't that hard, especially if you really understand how the units cancel out. But definitely email the TA about this.
- Sat Oct 06, 2018 3:26 pm
- Forum: SI Units, Unit Conversions
- Topic: formula units [ENDORSED]
- Replies: 69
- Views: 32773
Re: formula units [ENDORSED]
Wait, so when it is talking about formula units, it does not specifically mean atoms/molecules, right?

