Search found 60 matches
- Sun Mar 17, 2019 5:48 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Catalyst in rate law?
- Replies: 6
- Views: 8195
Re: Catalyst in rate law?
A catalyst can be present in the rate law if it is a reactant in the rate determining step. Because it is a reactant in that step, it is included in the rate law.
- Sun Mar 17, 2019 5:45 pm
- Forum: Balancing Redox Reactions
- Topic: How to balance half reaction
- Replies: 5
- Views: 836
Re: How to balance half reaction
I agree with Henry's answer except instead of adding OH- equal to double the amount of H2O initially added, find how many H one side needs to make it balanced and and add twice that amount of H2O to that side and that amount of OH- to the other side. Also don't forget to balance all the other reacta...
- Sun Mar 17, 2019 5:39 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: k' vs k'
- Replies: 3
- Views: 556
Re: k' vs k'
They are not related, they just happen to be written the same.
- Sun Mar 10, 2019 2:26 pm
- Forum: General Rate Laws
- Topic: Problem
- Replies: 1
- Views: 268
Re: Problem
t1/2 = 0.693/k
to get to 1/64 the original power, just do it 6 times because 2^6=64
to get to 1/64 the original power, just do it 6 times because 2^6=64
- Sun Mar 10, 2019 2:24 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Order
- Replies: 2
- Views: 336
Re: Order
Use experimental data to calculate the order of reactants in a reaction. Divide one experiment by another as shown in class on March 1st. The order shows how many of that molecule interact in the rate determining step.
- Sun Mar 10, 2019 2:23 pm
- Forum: General Rate Laws
- Topic: Unique rates
- Replies: 1
- Views: 239
Re: Unique rates
The two are equivalent, but typically when referring to the unique rate you would be referring to the equation that describes the rate with respect to one reactant or product while the rate law describes it in terms of all reactants. Eg. Unique rate = -\frac{1}{a}\frac{d[A]}{dt} for a as a reactant ...
- Sun Mar 03, 2019 8:27 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: concentration cell
- Replies: 2
- Views: 278
Re: concentration cell
A concentration cell is a galvanic cell with both sides containing the same solutions at different molarities. The Eo is always zero because it is calculated using the standard reduction potentials of both sides which are the same because the solutions contain the same components.
- Sun Mar 03, 2019 8:20 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Cell Diagram
- Replies: 4
- Views: 422
Re: Cell Diagram
Everything that is added to the solutions that participates in the redox reaction is put into the cell diagram except for water and the acid/base that is added to the solution to allow the reaction to happen. This means that the electrode, and oxidized/reduced materials are added, as well as other m...
- Sun Mar 03, 2019 8:16 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Concentration Cell
- Replies: 2
- Views: 316
Re: Concentration Cell
E o is always 0 for a concentration cell because E o refers to the potential of a solution at 1M concentration. For the concentration cell, E o would be calculated using 1M solutions of the solutions on each side, and since in a concentration cell, both solutions have the same components, the differ...
- Mon Feb 25, 2019 12:24 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Calculating cell potential using cathode and anode values
- Replies: 2
- Views: 247
Re: Calculating cell potential using cathode and anode values
Expanding on Aili's answer, the standard potential for the anode should be negative already because it is oxidized so it will be subtracted when you add them.
- Mon Feb 25, 2019 12:22 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Conductors and Cell Diagrams
- Replies: 1
- Views: 202
Re: Conductors and Cell Diagrams
An inert conductor is used when the ions on the cathode side will not form a solid on their own. This should be given I don't think we need to know when to use it and when not to.
- Mon Feb 25, 2019 12:17 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Hydrogen Electrode
- Replies: 1
- Views: 203
Re: Hydrogen Electrode
Comparing the half reactions is just one method for calculating the overall cell potential. The standard hydrogen electrode is an electrode that is used to measure the potentials of substances and to standardize them. A galvanic cell is set up and potential is measured between the cell of interest a...
- Tue Feb 19, 2019 1:08 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Molar Gibbs free energy
- Replies: 3
- Views: 636
Re: Molar Gibbs free energy
The molar Gibbs free energy refers to the amount of Gibbs free energy per mole of a substance.
Standard Gibbs free energy is the total amount of energy, not just the amount of energy in each mole of the substance.
Standard Gibbs free energy is the total amount of energy, not just the amount of energy in each mole of the substance.
- Tue Feb 19, 2019 1:05 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Van't Hoff Equation
- Replies: 3
- Views: 343
Re: Van't Hoff Equation
K is just a constant in the equation that relates entropy and enthalpy in a system.
If you want to know more I suggest you look at the section in the thermodynamics tab called Van't Hoff Equation.
If you want to know more I suggest you look at the section in the thermodynamics tab called Van't Hoff Equation.
- Tue Feb 19, 2019 1:02 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs free energy
- Replies: 7
- Views: 788
Re: Gibbs free energy
Gibbs free energy is a measure of energy contained within a system. More specifically, this energy is the energy in the system that can be accessed to do work. Enthalpy is the measure of energy due to temperature. Entropy is a measure of the number of quantum states that a system can occupy. Heat is...
- Sun Feb 10, 2019 1:32 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: 9.25 6th edition
- Replies: 1
- Views: 200
Re: 9.25 6th edition
Each SO2F2 can be in 1 of 6 configurations. Since we are calculating the molar entropy or entropy per mole, use the Boltzmann equation with n=6.022x1023
So,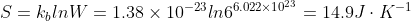
So,
- Sun Feb 10, 2019 1:26 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Calculation Help
- Replies: 1
- Views: 242
Re: Calculation Help
5/2 R should be used when pressure is constant and 3/2 R when volume is constant. They are derived from ideal gas equations not discussed in class.
- Sun Feb 10, 2019 1:23 pm
- Forum: Calculating Work of Expansion
- Topic: Using Derivative/Integral Equations
- Replies: 5
- Views: 576
Re: Using Derivative/Integral Equations
You won't have to integrate to solve any problems. They were just used to derive others from class.
As for knowing which equations to use when certain values are constant, you just have to remember which to use in which scenario.
As for knowing which equations to use when certain values are constant, you just have to remember which to use in which scenario.
- Sun Feb 03, 2019 4:09 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Enthalpy of Complicated Molecule
- Replies: 1
- Views: 218
Re: Enthalpy of Complicated Molecule
The way that you would find out the enthalpy of a molecule would be by using the enthalpies of the other molecules in the reaction which should be given. You could also add up the bond enthalpies if those are given.
- Sun Feb 03, 2019 1:16 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: HW problem 4A.11
- Replies: 1
- Views: 234
- Sat Feb 02, 2019 4:05 pm
- Forum: Calculating Work of Expansion
- Topic: SI unit for P
- Replies: 6
- Views: 698
Re: SI unit for P
In this class, use atm when reporting pressures.
- Fri Jan 25, 2019 10:55 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Energy of raising water temp, Adams Disc 1A
- Replies: 1
- Views: 211
Re: Energy of raising water temp, Adams Disc 1A
You should memorize the general pattern of rises and plateaus but not the specific numbers.
- Fri Jan 25, 2019 10:54 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: State properties
- Replies: 3
- Views: 362
Re: State properties
Adding on to that, because enthalpy is a state function, you can calculate it by adding the enthalpies of multiple reactions to find the enthalpy of the overall reaction.
- Fri Jan 25, 2019 10:51 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: methods
- Replies: 2
- Views: 264
Re: methods
You should know all three methods because you will need to choose one according to what you are given in each problem.
- Sat Jan 19, 2019 6:01 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: strong acids and bases
- Replies: 2
- Views: 407
Re: strong acids and bases
If you are given a K value, an acid with a Ka < 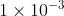 or a base with Kb less than that value will be a weak acid/base respectively.
or a base with Kb less than that value will be a weak acid/base respectively.
(This is also a general rule but works in the majority of situations)
(This is also a general rule but works in the majority of situations)
- Sat Jan 19, 2019 5:56 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Stability of reactions using equilibrium constant
- Replies: 1
- Views: 477
Re: Stability of reactions using equilibrium constant
The dissociation of F2 is more stable. The K value is larger meaning there is a higher ratio of products to reactants. The dissociated molecule is the product so there should be more products made in the F2 dissociation than the Cl2 dissociation.
- Sat Jan 19, 2019 5:53 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 7th Edit 5I.23
- Replies: 1
- Views: 70
Re: 7th Edit 5I.23
Did you make sure to use concentrations instead of moles of each chemical? (convert using moles / liters)
Also, in the Kc value don't forget to put the proper exponents on the terms that have coefficients in the balanced equation.
Also, in the Kc value don't forget to put the proper exponents on the terms that have coefficients in the balanced equation.
- Thu Jan 10, 2019 11:55 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5H3 from 7th edition
- Replies: 2
- Views: 110
Re: 5H3 from 7th edition
When the two equations are combined in full, the result will be: 2BrCl + H 2 + Cl 2 <--> Br 2 + 2HCl + Cl 2 To write the full equilibrium constant equation for this, we would get: K = \frac{[Br_{2}][2HCl][Cl_{2}]}{[2BrCl][H_{2}][Cl_{2}]} This is the same as both K values multiplied together. The Cl ...
- Thu Jan 10, 2019 11:24 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 7th Edition 5H.3
- Replies: 1
- Views: 77
7th Edition 5H.3
In this problem, both reactions were combined and their K values were multiplied together. Is this the case for all problems of this type or are there other factors to consider when combining the two K factors?
- Wed Jan 09, 2019 1:09 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kc for Gases
- Replies: 2
- Views: 143
Re: Kc for Gases
You are correct in saying that we use Kp for gases, but if necessary, you can convert using the ideal gas law: PV=nRT or P=(Concentration)RT
- Sun Dec 09, 2018 12:14 am
- Forum: Bronsted Acids & Bases
- Topic: 7th edition fundamentals J.9
- Replies: 1
- Views: 431
Re: 7th edition fundamentals J.9
The phosphoric acid will donate a H+ to each of 3 ammonia molecules. This will result in PO43- and 3NH4+. They come together to form the salt (NH4)3PO4.
- Sun Dec 09, 2018 12:12 am
- Forum: Bronsted Acids & Bases
- Topic: Fundamentals J.17 (7th Edition)
- Replies: 1
- Views: 293
Re: Fundamentals J.17 (7th Edition)
If the molecule dissociates into an ion that doesn't affect the pH such as Na+, it can be left out of the net ionic equation.
- Sun Dec 09, 2018 12:10 am
- Forum: Lewis Acids & Bases
- Topic: 12.25
- Replies: 1
- Views: 414
Re: 12.25
First calculate the amount of moles of Ba(OH) 2 using the molar mass. Then remember that it completely dissociates into that number of moles of Ba and twice the number of moles of OH. Divide by .1L to get the molarity of Ba and OH. Then calculate the pOH using the molarity of OH and subtract it from...
- Sun Dec 02, 2018 10:02 pm
- Forum: Hybridization
- Topic: Hybridization Application
- Replies: 2
- Views: 303
Re: Hybridization Application
Yes, whenever an atom is bonded to another atom, its regions of electron density are hybridized into sp, sp2, etc. orbitals.
- Sun Dec 02, 2018 9:55 pm
- Forum: Bronsted Acids & Bases
- Topic: Fundamentals J.1 Bronsted Acids and Bases
- Replies: 2
- Views: 419
Re: Fundamentals J.1 Bronsted Acids and Bases
There are certain characteristics to acids and bases that are good to remember. For example, a compound consisting of one hydrogen and one halogen will always be a strong acid. Molecules with group 1 or 2 metals and an OH group will be basic. Also, any compound with an N atom that has a lone pair wi...
- Sun Dec 02, 2018 9:42 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Polydentate ligand geometry
- Replies: 1
- Views: 227
Re: Polydentate ligand geometry
Polydentate ligand lone pairs are in the same orientation as atoms in the VSEPR model. For example, if there are 6 lone pairs from ligands bonding to the transition metal, they will be in an octahedral shape. The lone pairs should be the correct distance apart to form these shapes.
- Sun Nov 25, 2018 2:42 pm
- Forum: Hybridization
- Topic: BrF3
- Replies: 2
- Views: 1243
Re: BrF3
If you draw the Lewis structure, there should be 28 e - (21 from the 3 fluorine, 7 from bromine). The bromine is bonded to each of the 3 fluorine atoms by a single bond. That leaves 4 electrons which are put around the bromine atom as 2 lone pairs. This gives a total of 5 regions of electron density...
- Sun Nov 25, 2018 2:38 pm
- Forum: Hybridization
- Topic: Hybridization Orbitals
- Replies: 2
- Views: 441
Re: Hybridization Orbitals
The 1 and 2 after the H and C represent the 1 in 1s or 2 in 2sp shell respectively. This is to distinguish between other shells such as 2s, 3s, 4s, etc. or a 3sp hybrid orbital. The hydrogen has 1s because a H atom has a valence electron in the 1s shell. The C has 2sp because the orbitals are a hybr...
- Sun Nov 25, 2018 2:34 pm
- Forum: Hybridization
- Topic: Resonance and hybridization
- Replies: 1
- Views: 299
Re: Resonance and hybridization
In a resonance structure, the hybridization is found the same way as a non resonant structure. That is, by looking at the regions of electron density around the atom. If a lone pair in one resonance structure is a bonding pair in another resonance structure, the lone pair is considered to be in the ...
- Sun Nov 18, 2018 10:59 pm
- Forum: Bond Lengths & Energies
- Topic: Electrostatic Energy and Energy Density
- Replies: 1
- Views: 192
Re: Electrostatic Energy and Energy Density
I believe that we do not need to know these concepts for class.
- Sun Nov 18, 2018 10:36 pm
- Forum: Electronegativity
- Topic: Question about what makes something ionic
- Replies: 5
- Views: 497
Re: Question about what makes something ionic
Because the difference in their electronegativities is only about .76, the bond has mostly covalent character. Only bonds with a difference in electronegativity of 2 or greater are said to have mostly ionic character.
- Sun Nov 18, 2018 10:28 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angles in 7E 2E.13.b
- Replies: 4
- Views: 484
Bond Angles in 7E 2E.13.b
In this problem, the POCl 3 molecule has a tetrahedral shape. In class we learned that tetrahedral molecules always have bond angles of 109.5 o and this is the correct answer in the solutions manual. However, the bond angles are not exactly 109.5 because (I assume) of the double bond that the oxygen...
- Fri Nov 09, 2018 1:06 am
- Forum: Dipole Moments
- Topic: Dipole Equation- Units
- Replies: 1
- Views: 240
Re: Dipole Equation- Units
The unit for dipole moments is the debye which is 3.336x10-30 coulomb meter.
charge (coulombs) x distance (meters) = debye (coulomb meter)
charge (coulombs) x distance (meters) = debye (coulomb meter)
- Fri Nov 09, 2018 1:02 am
- Forum: Ionic & Covalent Bonds
- Topic: Bond strength and state of matter
- Replies: 2
- Views: 576
Re: Bond strength and state of matter
What determines if a substance is a solid, liquid, or gas is not necessarily bonds but interactions between the atoms/molecules. These can be hydrogen bonds, Van der Waals' interactions, etc. If these interactions are stronger, then the atoms/molecules will be held more tightly together so it will t...
- Thu Nov 08, 2018 9:11 pm
- Forum: Ionic & Covalent Bonds
- Topic: Ionic Solubility
- Replies: 2
- Views: 137
Re: Ionic Solubility
It has to do with the difference in electromagnetically between the two elements. Ag and F have very different electromagnetically so their bond has a high ionic character. This means that it will be soluble in water. As you go down the group, Cl, Br... the electronegativities get closer to that of ...
- Sun Nov 04, 2018 6:47 pm
- Forum: Electronegativity
- Topic: Problem 2D #11 (7th edition)
- Replies: 2
- Views: 331
Re: Problem 2D #11 (7th edition)
The polarizability gets bigger as the ion gets larger. The radius of O2- is less that the radius of N3-. Thus, it has a smaller polarizability. Also, N3- is less electronegative which also contributes.
- Sun Nov 04, 2018 6:44 pm
- Forum: Lewis Structures
- Topic: B9 in 7th Edition
- Replies: 1
- Views: 101
Re: B9 in 7th Edition
A) The brackets are just to distinguish between the Cl and NH4. B) The Molecule is K 3 P. The three K + ions surrounding the P 3- ion show that there are 3 potassium ions per phosphorous ion in this salt. The arrangement of K + ions around the phosphorous is arbitrary. C) Again here the brackets are...
- Sun Nov 04, 2018 6:38 pm
- Forum: Ionic & Covalent Bonds
- Topic: bond order
- Replies: 2
- Views: 2249
Re: bond order
The bond order is simply the number of bonds between two atoms.
For a single bond, the bond order is 1.
For a double bond, the bond order is 2.
etc.
For a single bond, the bond order is 1.
For a double bond, the bond order is 2.
etc.
- Sun Oct 28, 2018 9:19 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: 3d before 4s for Z > 10
- Replies: 3
- Views: 292
Re: 3d before 4s for Z > 10
This rule only comes into effect for elements above argon (Z=18). You are correct that 3d is written before 4s, etc. when writing electron configurations However the 4s shell will fill before the 3d shell. This can be seen for the elements Potassium and Calcium (19 & 20). K: [Ar] 4s 1 , Ca: [Ar]...
- Sun Oct 28, 2018 5:01 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: when 4s goes after 3d??
- Replies: 2
- Views: 626
Re: when 4s goes after 3d??
The 4s orbital is always written after the 3d orbitals because it has higher energy. Remember higher n means higher energy. However, The 4s orbital will fill before the 3d orbital which is why potassium and calcium have electrons in the 4s orbital but not the 3d orbital. Keep in mind the exceptions ...
- Sun Oct 28, 2018 4:36 pm
- Forum: Lewis Structures
- Topic: Lewis Dot Order
- Replies: 10
- Views: 1260
Re: Lewis Dot Order
It is generally best to choose the atom with the lowest ionization energy as the central atom. Start with this atom and arrange the others symmetrically around it.
- Sun Oct 21, 2018 4:50 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Atomic Spectra Post Module #13
- Replies: 1
- Views: 113
Re: Atomic Spectra Post Module #13
The answer is C.
Since the absorbed wavelengths are detected, the spectrometer can produce a spectrum with absorbed lines, hence the name absorption line spectrum. Because it is measuring absorbed lines, the experiment is called absorption spectroscopy.
Since the absorbed wavelengths are detected, the spectrometer can produce a spectrum with absorbed lines, hence the name absorption line spectrum. Because it is measuring absorbed lines, the experiment is called absorption spectroscopy.
- Sun Oct 21, 2018 4:47 pm
- Forum: Properties of Electrons
- Topic: Electrons - Wave Properties
- Replies: 4
- Views: 392
Re: Electrons - Wave Properties
All particles have particle and wave-like properties.
Electrons do have a wavelength and since they are small enough, the wavelength can usually be measured.
Larger objects such as a baseball have wavelengths that cannot be measured.
Electrons do have a wavelength and since they are small enough, the wavelength can usually be measured.
Larger objects such as a baseball have wavelengths that cannot be measured.
- Sun Oct 21, 2018 4:42 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Hw 1D.9 - radial nodes and angular nodal surfaces differences
- Replies: 1
- Views: 235
Re: Hw 1D.9 - radial nodes and angular nodal surfaces differences
We do not need to know radial nodes.
Angular nodal surfaces and nodal planes are the same so the question is asking for how many nodal planes in each.
Calculating them would involve the schrodinger equation which we are not using in class so just memorize them.
Angular nodal surfaces and nodal planes are the same so the question is asking for how many nodal planes in each.
Calculating them would involve the schrodinger equation which we are not using in class so just memorize them.
- Sun Oct 14, 2018 9:06 pm
- Forum: Photoelectric Effect
- Topic: 1B.15 Part C
- Replies: 2
- Views: 199
Re: 1B.15 Part C
Use the equation to find the energy of the incoming radiation: E_{photon}=E_{K}+\phi . The velocity of the ejected electron is given and the mass of the electron is in the list of constants, so you can calculate E_{K} (kinetic energy of electron) using \frac{1}{2}mv^2 . In part (b) you calculated th...
- Fri Oct 12, 2018 11:23 pm
- Forum: Photoelectric Effect
- Topic: Properties of Light
- Replies: 5
- Views: 344
Re: Properties of Light
The intensity of a source of light is the number of protons that come from the source. This is different from the energy of the light wave, which is dependent on the frequency of the wave, hence the equation: E=hv.
- Tue Oct 09, 2018 4:04 pm
- Forum: Properties of Light
- Topic: Problem 1B.5: Units KeV
- Replies: 2
- Views: 137
Re: Problem 1B.5: Units KeV
keV is a kilo-electron Volt, or 1000 electron volts. 1 Joule is equal to 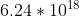 electron volts. So 1 Joule =
electron volts. So 1 Joule = 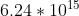 keV.
keV.
- Thu Oct 04, 2018 4:06 pm
- Forum: Significant Figures
- Topic: Molar Mass
- Replies: 5
- Views: 461
Re: Molar Mass
Use the most accurate molar mass given to you. If the periodic table you are using gives the molar mass of carbon as 12.011 g/mol, then use that. Because the problem you gave an example of starts with 3.45g carbon (3 sig figs), the final result should be rounded to 3 significant figures. In other wo...
- Wed Oct 03, 2018 1:38 pm
- Forum: Empirical & Molecular Formulas
- Topic: Writing formula from diagram
- Replies: 2
- Views: 343
Re: Writing formula from diagram
Look at how many atoms of each color there are and write the formula based off of the key it gives you.
Eg. F1 (7th edition). Dark grey = C, White = H, Red = O
10 Dark grey atoms
16 white atoms
1 red atom
Molecular formula - C10H16O
The order in which you put the atoms doesn't really matter.
Eg. F1 (7th edition). Dark grey = C, White = H, Red = O
10 Dark grey atoms
16 white atoms
1 red atom
Molecular formula - C10H16O
The order in which you put the atoms doesn't really matter.
- Mon Oct 01, 2018 2:38 pm
- Forum: SI Units, Unit Conversions
- Topic: Fundamental Exercises E3 [ENDORSED]
- Replies: 3
- Views: 259
Re: Fundamental Exercises E3 [ENDORSED]
I agree with Matthew. Because the question says that the lab can manipulate individual atoms, it is safe to assume that each circle on the scale represents a single atom. Therefore, the answer will be 3 atoms of astatine rather than 3 moles of astatine.

