Search found 32 matches
- Fri Dec 07, 2018 10:53 pm
- Forum: Biological Examples
- Topic: What should we know for the final?
- Replies: 9
- Views: 1034
What should we know for the final?
Are there any specific biological examples that we need to know for the final? In terms of structure, function, etc.
- Sun Dec 02, 2018 4:55 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Final [ENDORSED]
- Replies: 10
- Views: 1356
Re: Final [ENDORSED]
I feel like there might be a practice final because Lyndon, who did the Garlic Bread midterm questions last time, has a session on Friday. He might tell us in class during one of the lectures this week. Also, I assume that the TAs and UAs will also go over practice final questions during their own s...
- Wed Nov 28, 2018 3:35 pm
- Forum: Ionic & Covalent Bonds
- Topic: Ionic/Covalent bond cut offs
- Replies: 1
- Views: 307
Ionic/Covalent bond cut offs
What are the electronegativity cut offs that can be used to determine if a bond is ionic/covalent?
- Sun Nov 25, 2018 7:03 pm
- Forum: Properties of Light
- Topic: Speed of light
- Replies: 13
- Views: 3898
Speed of light
When doing calculations, should we use 2.99792 × 108 m.s-1 or 3.00 × 108 m.s-1 for the speed of light?
I used the 3.00 × 108 m.s-1 on the midterm and got an answer that was two hundredths off and got it marked wrong, so what should be the accurate number to use?
I used the 3.00 × 108 m.s-1 on the midterm and got an answer that was two hundredths off and got it marked wrong, so what should be the accurate number to use?
- Sun Nov 25, 2018 6:58 pm
- Forum: Limiting Reactant Calculations
- Topic: Finding limiting reagent
- Replies: 6
- Views: 827
Finding limiting reagent
What is the easiest/quickest way to determine what the limiting reagent in a chemical reaction is?
- Sun Nov 25, 2018 6:56 pm
- Forum: Hybridization
- Topic: Hybridization scheme
- Replies: 3
- Views: 332
Hybridization scheme
What's the easiest way to determine hybridization schemes for certain molecules?
- Fri Nov 16, 2018 11:48 am
- Forum: Sigma & Pi Bonds
- Topic: Significance of sigma and pi bonds
- Replies: 2
- Views: 384
Significance of sigma and pi bonds
What's the significance of sigma and pi bonds? How are they useful to know when looking at molecular interactions?
- Fri Nov 16, 2018 11:27 am
- Forum: Hybridization
- Topic: General definition/context
- Replies: 2
- Views: 338
General definition/context
What is hybridization in general, in terms of its definition as well as how it is used in the context of this lesson?
- Fri Nov 16, 2018 9:38 am
- Forum: Formal Charge and Oxidation Numbers
- Topic: Shortcut
- Replies: 4
- Views: 988
Re: Shortcut
The shortcut is basically to first count how many electrons are surrounding a single atom. For example, when looking at the formal charge for Cl in PCl 5 , you would count that there are 6 lone electrons and then one electron right next to the Cl that is being shared with the P (in other words, only...
- Sun Nov 11, 2018 8:38 pm
- Forum: Bond Lengths & Energies
- Topic: Determine bond length
- Replies: 3
- Views: 470
Determine bond length
Is there a way to determine bond length of a Lewis structure with single/double/triple bonds? Or does that information have to be given to us in the problem?
- Sun Nov 04, 2018 9:05 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: When to multiply by 2
- Replies: 5
- Views: 968
When to multiply by 2
When do we multiply the uncertainty in p or x or v by 2? I'm getting confused when doing calculations.
- Sun Nov 04, 2018 8:46 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Post-Module Q#18
- Replies: 3
- Views: 689
Re: Post-Module Q#18
The radius of the atom represents 1/2 of the uncertainty of the position of the electron, the diameter equaling the entire uncertainty. the size of the atom represents the uncertainty in position. You can use this uncertainty, as well as the mass of an electron and the constant h/4pi to calculate d...
- Sun Nov 04, 2018 6:21 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Post-Module Q#18
- Replies: 3
- Views: 689
Post-Module Q#18
The question states: The hydrogen atom has a radius of approximately 0.05 nm. Assume that we know the position of an electron to an accuracy of 1 % of the hydrogen radius, calculate the uncertainty in the speed of the electron using the Heisenberg uncertainty principle. Comment on your value obtaine...
- Sun Nov 04, 2018 6:09 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Post-Module Q#17
- Replies: 1
- Views: 331
Post-Module Q#17
The question states: Calculate the uncertainty (indeterminacy) in position of an electron if its uncertainty in speed is one hundredth the speed of light.
Does this mean the uncertainty is 3x10^6? if so, does delta v = 2(3x10^6)?
Does this mean the uncertainty is 3x10^6? if so, does delta v = 2(3x10^6)?
- Sat Nov 03, 2018 8:29 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Post-Module Q#42
- Replies: 1
- Views: 355
Post-Module Q#42
The question asks: An excited hydrogen atom emits light with a frequency of 1.14 x 10^14 Hz to reach the energy level n = 4. In what principle quantum level did the electron begin?
What is the easiest way to solve this?
What is the easiest way to solve this?
- Sat Nov 03, 2018 11:52 am
- Forum: Properties of Light
- Topic: Photoelectric Effect - waves vs. photons
- Replies: 3
- Views: 631
Photoelectric Effect - waves vs. photons
In the photoelectric effect, do we look at the light as waves or photons? I keep getting confused with questions asking for example: if long wavelength light is not ejecting e- from a surface, will increasing intensity of light result in e- being ejected?? I think increasing the intensity of light w...
- Fri Nov 02, 2018 9:05 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Sig figs???
- Replies: 1
- Views: 348
Sig figs???
When there's a problem asking for the change in energy from n=3 to n=1 of a hydrogen atom, how many sig figs should our answer have, since there's only one sig fig from the given data?
- Mon Oct 29, 2018 9:26 am
- Forum: Ionic & Covalent Bonds
- Topic: Roman numerals next to element
- Replies: 8
- Views: 6158
Re: Roman numerals next to element
Roman numerals are used to name the different ions of the transition metals. An example would be: Cu3+ named as Cu(III).
- Mon Oct 29, 2018 9:23 am
- Forum: Trends in The Periodic Table
- Topic: Periodic table
- Replies: 4
- Views: 536
Re: Periodic table
I know that ionization energy is the energy needed to remove an electron from an atom. The further away the electron is from the nucleus, the easier it is to remove the electron. Ionization energies decrease down a group and increase across a period.
- Mon Oct 29, 2018 9:17 am
- Forum: Quantum Numbers and The H-Atom
- Topic: Finding number of elements that have given quantum numbers
- Replies: 1
- Views: 296
Finding number of elements that have given quantum numbers
What is the easiest way to find the number of elements that have given quantum numbers?
Example: given n = 5 and l = 1, how many elements can have these quantum numbers?
Example: given n = 5 and l = 1, how many elements can have these quantum numbers?
- Mon Oct 29, 2018 9:12 am
- Forum: Properties of Light
- Topic: Photoelectric Effect
- Replies: 7
- Views: 937
Re: Photoelectric Effect
As the light source is increased in intensity, then the number of electrons ejected increases. Light intensity is proportional to the number of electrons ejected.
- Mon Oct 29, 2018 9:09 am
- Forum: Significant Figures
- Topic: Sig Figs in the Middle of a Problem
- Replies: 9
- Views: 2501
Re: Sig Figs in the Middle of a Problem
It is better to round off at the end of the problem, instead of in the middle of a calculation, to ensure accuracy. Use as many digits as possible in the middle of a calculation and just carry those numbers over until you're at the end of the problem.
- Wed Oct 24, 2018 9:02 pm
- Forum: Properties of Light
- Topic: SI Units
- Replies: 6
- Views: 618
Re: SI Units
Should we also know conversions like M (mega)? It was on the homework so I'm just wondering
- Sun Oct 21, 2018 10:47 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Test Question
- Replies: 8
- Views: 749
Re: Test Question
It's good to know them for context and conceptual understanding, but we don't have to know how to draw them.
- Sun Oct 21, 2018 10:44 pm
- Forum: Properties of Light
- Topic: Test 2 Equations
- Replies: 14
- Views: 1090
Test 2 Equations
What are the equations we should memorize for Test 2?
- Sun Oct 21, 2018 10:42 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Lyman, Balmer, Pascal
- Replies: 10
- Views: 1459
Re: Lyman, Balmer, Pascal
Do we have to memorize these for the test this week?
- Fri Oct 12, 2018 8:23 pm
- Forum: SI Units, Unit Conversions
- Topic: formula units [ENDORSED]
- Replies: 69
- Views: 32898
Re: formula units [ENDORSED]
I looked at formula units as the number of atoms/molecules, depending on what is given. I used Avogadro's number to find the formula units asked for in the problem.
- Fri Oct 12, 2018 8:22 pm
- Forum: Limiting Reactant Calculations
- Topic: Test 1
- Replies: 8
- Views: 1155
Re: Test 1
The test was fairly straightforward, and most of the problems were similar to the homework problems. It was also pretty short, and most of us finished early, giving us time to even check back on all of the problems again.
- Mon Oct 08, 2018 10:45 pm
- Forum: Limiting Reactant Calculations
- Topic: How to find the limiting reactant easily and quickly
- Replies: 5
- Views: 13823
How to find the limiting reactant easily and quickly
I'm having trouble finding easy ways to determine the limiting reactant. Any advice??? There is also a problem in one of the post-assessment modules that asks questions about the chemical equation 2A + 1B --> 3C, such as what reactant is in excess and what is the maximum amount of product that can b...
- Fri Oct 05, 2018 9:24 am
- Forum: Limiting Reactant Calculations
- Topic: When to use H vs H2
- Replies: 7
- Views: 1105
Re: When to use H vs H2
I believe you do multiply by 2 when using H2. Because there are two atoms of hydrogen, you would multiply the molar mass of 1 H atom by 2 to get the total molar mass of H2.
- Fri Oct 05, 2018 9:10 am
- Forum: Balancing Chemical Reactions
- Topic: Post-Module Assessment typo?? - Balancing Chemical Reactions
- Replies: 3
- Views: 438
Post-Module Assessment typo?? - Balancing Chemical Reactions
On question #17, it asks us to balance the equation  .
.
I believe the correct answer is C)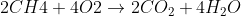 , but the survey says it is wrong, maybe it's a typo?
, but the survey says it is wrong, maybe it's a typo?
I believe the correct answer is C)
- Fri Oct 05, 2018 9:01 am
- Forum: Balancing Chemical Reactions
- Topic: How To....
- Replies: 16
- Views: 2638
Re: How To....
I believe Dr. Lavelle said we should include the state of matter when balancing an equation. We will probably be able to recognize the states of matter as we go along and do problems, so I think we will pick it up eventually. If not, then the state of matter will be stated in the question. To balanc...

