Search found 90 matches
- Sun Mar 17, 2019 8:44 am
- Forum: Administrative Questions and Class Announcements
- Topic: LYNDON'S PORK RAMEN REVIEW
- Replies: 37
- Views: 7569
Re: LYNDON'S PORK RAMEN REVIEW
For question 14 part (c). What information is needed to determine the order of each step?
- Sat Mar 16, 2019 10:17 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 7th 6.65
- Replies: 2
- Views: 464
Re: 7th 6.65
I used 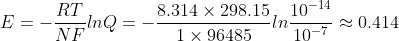 . I thought Q is between 10^-7 and 10^7, but the answer seems to imply that instead it should be between 10^-14 and 10^14.
. I thought Q is between 10^-7 and 10^7, but the answer seems to imply that instead it should be between 10^-14 and 10^14.
- Wed Mar 13, 2019 9:14 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 7th 6.65
- Replies: 2
- Views: 464
7th 6.65
Can anyone tell me how to arrive at this answer?
- Mon Mar 11, 2019 7:09 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: reducing power and oxidizing power
- Replies: 2
- Views: 502
Re: reducing power and oxidizing power
The more positive the reduction potential, the stronger the oxidizing power and the weaker the reducing power. Just think reduction potential as measuring a compound's tendency to be reduced. A more positive reduction potential means that itself is more easily reduced than oxidized.
- Mon Mar 11, 2019 7:06 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Frequency factor A
- Replies: 7
- Views: 810
Re: Frequency factor A
In the book, A is just called the pre-exponential factor. I think it will become clearer in the next lecture as we get into collision theory. Then you see what constants are included in A.
- Mon Mar 11, 2019 6:45 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode positive or negative in galvanic cell
- Replies: 1
- Views: 318
Re: Anode positive or negative in galvanic cell
First, a galvanic cell is not connected to a battery. A galvanic cell is a cell that generates electricity through a spontaneous chemical reaction. A cell that is connected to a battery is an electrolytic cell. An electrolytic cell uses energy from the battery to drive reactions. Then to answer your...
- Tue Mar 05, 2019 3:57 pm
- Forum: General Rate Laws
- Topic: how to calculate reaction rates
- Replies: 2
- Views: 395
Re: how to calculate reaction rates
Use any formula from rate law, integrated rate law, and half-life. Taking a look at the examples in the textbook is pretty helpful.
- Tue Mar 05, 2019 3:47 pm
- Forum: First Order Reactions
- Topic: Rate constant and half life
- Replies: 3
- Views: 454
Re: Rate constant and half life
Because /k) for first-order reactions.
for first-order reactions.
- Tue Mar 05, 2019 3:44 pm
- Forum: First Order Reactions
- Topic: Radioactive Decay
- Replies: 3
- Views: 359
Re: Radioactive Decay
Because it has a constant half-life. All first-order reactions have constant half-lives.
- Tue Feb 26, 2019 7:55 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Cell potential
- Replies: 2
- Views: 337
Re: Cell potential
Can you specify what you mean by changing the "stoichiometric coefficient of one reactant changes"? Will the equation still be balanced if you change only one reactant's coefficient?
- Mon Feb 25, 2019 2:29 pm
- Forum: Van't Hoff Equation
- Topic: Derivation
- Replies: 3
- Views: 668
Re: Derivation
From the 7th ed textbook page 433.
- Mon Feb 25, 2019 2:24 pm
- Forum: Balancing Redox Reactions
- Topic: 7th edition 6L.7, Adams, Disc 1A
- Replies: 2
- Views: 387
Re: 7th edition 6L.7, Adams, Disc 1A
For the same question, how is the right-half cell set up with three solids?
- Fri Feb 22, 2019 11:31 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Reactants As Electrodes
- Replies: 1
- Views: 248
Reactants As Electrodes
In some half-cells the solid reactant can be used as the electrode, such as zinc in Zn/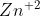 . How do I know which solid reactants can be used as electrodes and which ones cannot?
. How do I know which solid reactants can be used as electrodes and which ones cannot?
- Fri Feb 22, 2019 10:47 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Porous Disk
- Replies: 1
- Views: 316
Porous Disk
I have two questions about porous disks. 1. When a porous disk is used, why do electrons flow through electrodes instead of through the solution to the other half-cell? 2. When a porous disk is used, are the reactants mixed? If they are, why don't they react directly without donating electrons to an...
- Thu Feb 21, 2019 2:48 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Standard Cell Potential
- Replies: 2
- Views: 392
Re: Standard Cell Potential
When it's above 0, it means that the flow of electron from the left half-cell to the right half-cell is spontaneous. That is, oxidation in the left half-cell and reduction in the right half-cell.
- Thu Feb 21, 2019 2:43 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Inert Electrodes
- Replies: 2
- Views: 324
Re: Inert Electrodes
I think platinum is often used as an inert electrode because it does not react with the reactants in the cell. They are only responsible for conduction of current. An example is a hydrogen half-cell with uses platinum as the electrode.
- Thu Feb 21, 2019 8:55 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy Meaning
- Replies: 6
- Views: 751
Re: Gibbs Free Energy Meaning
G=H-TS. Gibbs free energy is the energy available to do work. It's less than the energy of a system because not all of the energy can be used to do work.
- Tue Feb 12, 2019 10:26 pm
- Forum: Calculating Work of Expansion
- Topic: Uses of different formulas
- Replies: 3
- Views: 438
Re: Uses of different formulas
If the external pressure is constant, it's irreversible expansion.
- Sun Feb 10, 2019 10:18 pm
- Forum: Calculating Work of Expansion
- Topic: Reversible Expansion
- Replies: 3
- Views: 433
Re: Reversible Expansion
It depends on the system at hand. If the system is isolated, heat cannot come into the reversible expansion and replace the additional energy loss, so the first law of thermodynamics tells us that in the case that both systems (reversible and irreversible) are isolated, the irreversible one would i...
- Sun Feb 10, 2019 10:11 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Contribution of Motion to Internal Energy
- Replies: 1
- Views: 356
Contribution of Motion to Internal Energy
Have trouble getting the right answer of Self-test 4B.3A on the 7th ed book. "Estimate the contribution of motion to the molar internal energy of water vapor at 25 Celcius degrees." Why is it not 3.72 kJ.Mol^-1?
- Sun Feb 10, 2019 9:21 pm
- Forum: Calculating Work of Expansion
- Topic: Reversible Work and Maximum Work
- Replies: 5
- Views: 543
Re: Reversible Work and Maximum Work
The expansion for a reversible system is done infinitely slowly and less heat is released to the surroundings resulting in more work done. You can better understand this process by viewing the pressure volume graphs for the two types of systems. The area under the curves shows you the work done by ...
- Sun Feb 10, 2019 9:17 pm
- Forum: Calculating Work of Expansion
- Topic: Reversible Expansion
- Replies: 3
- Views: 433
Reversible Expansion
I understand mathematically and graphically why a reversible expansion does more work, but does gas undergoing irreversible expansion ends up with more internal energy because it does less work?
- Tue Feb 05, 2019 4:57 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: heat capacity
- Replies: 2
- Views: 352
Re: heat capacity
I think they are the same, except the enthalpy ones are often in J per mol while specific heat capacity can be in J per mole or J per gram. The value of the enthalpy ones can also change when you change the stoichiometry of the reaction of vaporization/sublimation.
- Tue Feb 05, 2019 4:18 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: ΔH and ΔU
- Replies: 6
- Views: 879
Re: ΔH and ΔU
And if concentration is concerned, it would be 1M.
- Tue Feb 05, 2019 4:14 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Microstate
- Replies: 7
- Views: 820
Re: Microstate
According to Boltzmann’s equation S=k*ln(W), where W is the degeneracy, what I understand to be the number of possible microstates of the same energy level, more the number of microstates with the same energy level higher the entropy value.
- Tue Feb 05, 2019 4:09 pm
- Forum: Calculating Work of Expansion
- Topic: Work on Phases of Matter
- Replies: 2
- Views: 383
Re: Work on Phases of Matter
I think any system changes energy when work is done on it.
- Mon Jan 28, 2019 4:59 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Delta H
- Replies: 4
- Views: 497
Re: Delta H
An increase in T means that the surroundings have taken up some energy given out by the reaction. If a reaction system gives off energy then its own energy content has decreased, hence a decrease in enthalpy (H).
- Mon Jan 28, 2019 4:55 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Surface area and heat capacity [ENDORSED]
- Replies: 2
- Views: 309
Re: Surface area and heat capacity [ENDORSED]
I think a large surface area allows a quicker rise in temperature instead of a larger one. Which means the magnitude of the increase in temperature would still be the same when two objects of different surface areas are given the same amount of thermal energy.
- Mon Jan 28, 2019 4:49 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Neutralization reactions
- Replies: 6
- Views: 850
Re: Neutralization reactions
I think it's always exothermic because the only change in bonds is the formation of water molecules. Since the other ions are dissolved in water, they don't have a change in bonds.
- Sat Jan 26, 2019 10:56 pm
- Forum: Phase Changes & Related Calculations
- Topic: Phase Changes and Temperature
- Replies: 3
- Views: 373
Re: Phase Changes and Temperature
Heat energy is used to break intermolecular bonds, so kinetic energy stays constant.
- Fri Jan 25, 2019 3:42 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: bond enthalpies
- Replies: 5
- Views: 565
Re: bond enthalpies
Yes, because breaking a bond is always endothermic.
- Fri Jan 25, 2019 3:37 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Strength and Weakness
- Replies: 12
- Views: 1120
Re: Strength and Weakness
If possible, you can compare their X-H bond strength to see which one is more likely to lose protons. That would be the stronger acid.
- Fri Jan 25, 2019 3:34 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: What not to count
- Replies: 9
- Views: 911
Re: What not to count
Do you mean what not to include in equilibrium constant formula? If so then you should just disregard anything in solid and liquid state.
- Mon Jan 21, 2019 9:17 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Ka1 Ka2
- Replies: 3
- Views: 521
Re: Ka1 Ka2
Ka2 would always be smaller than Ka1 because it's harder to lose a proton from an already positively charged molecule.
- Mon Jan 21, 2019 9:15 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: how to determine if something is an acid or base
- Replies: 4
- Views: 388
Re: how to determine if something is an acid or base
A base tends to take on a proton, so having an OH group does not make it a base. You can try to memorize the names and formulae of common acids and bases. They are listed somewhere in the Fundamentals part of the book if you are using the 7th edition.
- Mon Jan 21, 2019 9:08 pm
- Forum: Administrative Questions and Class Announcements
- Topic: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
- Replies: 179
- Views: 23707
Re: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
Don't we need the volume to solve question 5 on worksheet 1?
- Mon Jan 21, 2019 8:40 pm
- Forum: Administrative Questions and Class Announcements
- Topic: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
- Replies: 179
- Views: 23707
Re: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
lindsey_ammann_4E wrote:Meigan Wu 2E wrote:On Worksheet 2, why is the concentration of CO3 the same as Ka2 for problem 4b?
^ I have the same question
Because some approximation was used. You can look at the example on page 488 on the 7th edition textbook.
- Tue Jan 15, 2019 8:52 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Hydrofluoric Acid
- Replies: 3
- Views: 378
Hydrofluoric Acid
Is hydrofluoric acid a strong acid?
- Tue Jan 15, 2019 5:20 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 11.43 6th edition
- Replies: 4
- Views: 461
Re: 11.43 6th edition
Do you know the volume? I think an ICE box can be used for partial pressure.
- Tue Jan 15, 2019 5:13 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Temperature
- Replies: 3
- Views: 314
Re: Temperature
I think we should always use Kelvin unless you are just using the temperature difference.
- Tue Jan 15, 2019 5:11 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Adding Equations
- Replies: 3
- Views: 407
Re: Adding Equations
You can multiply the equilibrium constants of the two reactions to get that of the third reaction.
- Fri Jan 11, 2019 6:23 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: 6th Edition 11.115
- Replies: 1
- Views: 257
Re: 6th Edition 11.115
Yes, we should only consider the number of moles of gases on each side in case of a change in pressure.
- Fri Jan 11, 2019 6:19 pm
- Forum: Ideal Gases
- Topic: Definition of Ideal Gases?
- Replies: 3
- Views: 360
Definition of Ideal Gases?
What defines ideal gases?
- Fri Jan 11, 2019 6:14 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kp vs Kc
- Replies: 6
- Views: 622
Re: Kp vs Kc
In a reaction that only involves gases, Kp is much more useful than Kc.
- Wed Dec 05, 2018 7:26 pm
- Forum: Lewis Acids & Bases
- Topic: Identifying Lewis Acids and Bases
- Replies: 3
- Views: 1171
Identifying Lewis Acids and Bases
Question 6.5 in 7th edition asks that, in the reaction between hydrogen peroxide H2O2 and sulfur trioxide SO3 to form H2SO5, which reactant is Lewis acid and which is base. Can anyone explain why SO2 is the acid and H2O2 the base?
- Wed Dec 05, 2018 6:35 pm
- Forum: Polyprotic Acids & Bases
- Topic: Defintion
- Replies: 39
- Views: 2543
Re: Defintion
when it can donate or accept more than one proton
- Wed Dec 05, 2018 6:31 pm
- Forum: Lewis Acids & Bases
- Topic: Weak Acids and Bases 12.51
- Replies: 2
- Views: 666
Re: Weak Acids and Bases 12.51
Can anyone also explain question (d)? I know that HClO4 is a strong acid and H3PO4 is not, but why is H3PO4 weaker?
- Tue Dec 04, 2018 11:09 am
- Forum: Amphoteric Compounds
- Topic: amphoteric substances
- Replies: 3
- Views: 415
Re: amphoteric substances
Oxides formed by metalloids in the diagonal band are often amphoteric, such as barium oxides, aluminum oxides, gallium (Ga) oxides, tin (Sn) oxides, antimony (Sb) oxides and lead oxides.
- Wed Nov 28, 2018 11:21 pm
- Forum: Hybridization
- Topic: Sulfur Trioxide Hybridization
- Replies: 4
- Views: 5220
Re: Sulfur Trioxide Hybridization
I guess I wasn't clear enough about my question. I do understand the relationship between steric number and type of hybridization. What I am trying to ask is what (hybrid) orbitals is the sulfur atom using to form the three pi bonds. Normally pi bonds are formed by p orbitals, but in this case, only...
- Wed Nov 28, 2018 11:10 pm
- Forum: Hybridization
- Topic: Sulfur Trioxide Hybridization
- Replies: 4
- Views: 5220
Re: Sulfur Trioxide Hybridization
If you look at the Lewis structure of this molecule, a central S atom is bonded to 3 O atoms. Each S-O bond is a double bond, and S has no lone pairs. Thus, S has 3 regions of electron density (a double bond counts as a single region of electron density, and there are 3 of them, hence 3 regions of ...
- Wed Nov 28, 2018 9:31 pm
- Forum: Hybridization
- Topic: Sulfur Trioxide Hybridization
- Replies: 4
- Views: 5220
Sulfur Trioxide Hybridization
Why is SO3 sp2 hybridized? If two of the three p orbitals are used in hybridization, how are the three pi bonds formed with only one unhybridized p orbital left? Can d orbitals form pi bonds with p orbitals?
- Wed Nov 28, 2018 7:37 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: N2O Lewis Structure
- Replies: 2
- Views: 534
Re: N2O Lewis Structure
I think the less electronegative one should be in the middle because they more readily share electrons.
- Wed Nov 28, 2018 2:39 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: TM charge
- Replies: 4
- Views: 477
Re: TM charge
Yes, the chlorine ions are bound to the metal. The metal forms ionic bonds with chlorine ions and coordinate covalent bonds with ligands. You can consider this as adding a few ligands to an ionic compound.
- Wed Nov 28, 2018 2:31 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Seesaw Bond Angles
- Replies: 6
- Views: 16110
Re: Seesaw Bond Angles
the shape should look like this
- Wed Nov 28, 2018 2:27 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: ClO2+
- Replies: 7
- Views: 1628
Re: ClO2+
Shouldn't the molecule be linear? If the chlorine atom has two lone pairs and two bonding pairs. if there are two lone pairs and two bonding pairs it should be bent. I know it sounds weird bc the two bonding and lone "cancel" each other out but if you think about it, we all know the shape...
- Tue Nov 13, 2018 8:27 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: ClO2+
- Replies: 7
- Views: 1628
Re: ClO2+
Shouldn't the molecule be linear? If the chlorine atom has two lone pairs and two bonding pairs.
- Tue Nov 13, 2018 8:24 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Difference between molecular shape & shape
- Replies: 2
- Views: 310
Re: Difference between molecular shape & shape
I think they are the same. We always consider lone pairs when determining the shape of a molecule, even if we kind of ignore them when naming the shape. So an angular molecule is never trigonal planar whether we are talking about "shape" or "molecular shape".
- Tue Nov 13, 2018 8:14 pm
- Forum: Dipole Moments
- Topic: Determining Intermolecular Forces
- Replies: 5
- Views: 3352
Re: Determining Intermolecular Forces
Try to determine whether the molecule is ionic, polar covalent, or non-polar covalent. Ionic compounds have ion-ion interaction. Polar molecules have polar-polar or hydrogen bonding. And, I think, all three have London dispersion forces.
- Sat Nov 10, 2018 4:19 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR?
- Replies: 9
- Views: 973
Re: VSEPR?
Lewis structure only considered valence electrons, while VSEPR also takes minimization of electron repulsion into account.
- Thu Nov 08, 2018 9:17 am
- Forum: Administrative Questions and Class Announcements
- Topic: Test during Thanksgiving Week?
- Replies: 2
- Views: 344
Re: Test during Thanksgiving Week?
The newest version of test schedule on the website says "Starting Tuesday November 27", though the version I downloaded earlier says November 20.
- Thu Nov 08, 2018 9:08 am
- Forum: Bond Lengths & Energies
- Topic: Interaction Potential Energy equation
- Replies: 2
- Views: 373
Re: Interaction Potential Energy equation
E_p∝−(α_1 α_2)/r^6
- Mon Nov 05, 2018 9:23 am
- Forum: Lewis Structures
- Topic: ONF
- Replies: 2
- Views: 390
Re: ONF
I think this is just an exception. Nitrogen is in the center because that gives the lowest formal charges of all three atoms.
- Fri Nov 02, 2018 6:48 pm
- Forum: Lewis Structures
- Topic: valence electrons in d block
- Replies: 1
- Views: 314
Re: valence electrons in d block
Valence electrons refer to the outermost electron shell, which also has the highest quantum number (n). So for d-block elements, I think we just need to show the 2 electrons in the outermost s orbital. Don't worry about the outermost d orbital because it's at a lower energy level, with a smaller qua...
- Wed Oct 31, 2018 11:58 am
- Forum: Formal Charge and Oxidation Numbers
- Topic: Stable Condition
- Replies: 8
- Views: 2571
Re: Stable Condition
I think a molecule is the most stable when the formal charge of each atom, instead of the whole molecule, is zero. In SO4 we can't organize electrons in the way that every atom has a formal charge of 0, and the SO4 with a 2- charge is the most stable one we can get which has a structure with least f...
- Wed Oct 31, 2018 11:46 am
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal CHarge
- Replies: 5
- Views: 498
Re: Formal CHarge
You'd have to check every single atom in the molecule because the molecule is the most stable when each atom in it has a formal charge of 0. You can calculate the formal charge of an atom using the formula [# of valence electrons] - [# of bonded electrons/2] - [# of non-bonding electrons]
- Wed Oct 31, 2018 11:39 am
- Forum: Trends in The Periodic Table
- Topic: Electron Affinity equation/definition
- Replies: 2
- Views: 431
Re: Electron Affinity equation/definition
So stronger the electron affinity, larger the value of Eea, because X would be fairly unstable with high energy right?
- Sat Oct 27, 2018 3:07 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Heisenberg- In relation to wavelength
- Replies: 7
- Views: 737
Re: Heisenberg- In relation to wavelength
Because of λ=h/p, an increase in uncertainty of p increases the uncertainty of λ.
- Wed Oct 24, 2018 8:40 pm
- Forum: Administrative Questions and Class Announcements
- Topic: DOWNLOAD SESSION WORKSHEETS HERE - Sundays 4-6pm (Karen) [ENDORSED]
- Replies: 135
- Views: 39122
Re: DOWNLOAD SESSION WORKSHEETS HERE - Sundays 4-6pm (Karen) [ENDORSED]
Thank you so much Karen :)
- Tue Oct 23, 2018 10:37 pm
- Forum: DeBroglie Equation
- Topic: deriving DeBroglie
- Replies: 2
- Views: 278
Re: deriving DeBroglie
• for a photon: E=pc, E=hv
• ∴pc=hc/λ → λ=hp
• ∴pc=hc/λ → λ=hp
- Tue Oct 23, 2018 11:24 am
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Indeterminacy Equation use
- Replies: 2
- Views: 329
Re: Indeterminacy Equation use
You need to divide deltaX by 2 to get the +/- value.
- Mon Oct 22, 2018 9:49 am
- Forum: Einstein Equation
- Topic: 7th Edition Book, Section 1.A #9
- Replies: 2
- Views: 571
Re: 7th Edition Book, Section 1.A #9
four events are mentioned in the question, you just need to infer the type of electromagnetic wave in each row using the wavelength you get, and then knowing the type of wave gives you the event
- Mon Oct 22, 2018 9:45 am
- Forum: *Particle in a Box
- Topic: Test 2
- Replies: 9
- Views: 1372
Re: Test 2
yes
- Mon Oct 22, 2018 9:44 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: 7th Edition Book, Section 1.D #23
- Replies: 2
- Views: 164
Re: 7th Edition Book, Section 1.D #23
the 4 orbitals are 2s, 2px, 2py, and 2pz
- Fri Oct 19, 2018 10:05 am
- Forum: DeBroglie Equation
- Topic: Energy=pc
- Replies: 4
- Views: 551
Re: Energy=pc
I'm confused, when is mass squared in 1/2 pc? Isn't that for electrons, 1/2 pc=1/2 mv*c=1/2 mv*v?
- Thu Oct 18, 2018 7:55 pm
- Forum: Properties of Light
- Topic: Do I have to memorize the spectrum?
- Replies: 20
- Views: 1684
Re: Do I have to memorize the spectrum?
No, you don't.
- Thu Oct 18, 2018 2:56 pm
- Forum: DeBroglie Equation
- Topic: Energy=pc
- Replies: 4
- Views: 551
Energy=pc
KE=1/2 mv^2, but why is Energy=pc true instead of Energy=1/2 pc?
- Thu Oct 18, 2018 2:30 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Nodes/ Nodals
- Replies: 2
- Views: 248
Re: Nodes/ Nodals
What's a nodal 0?
- Mon Oct 15, 2018 12:29 pm
- Forum: DeBroglie Equation
- Topic: Mass of electrons
- Replies: 4
- Views: 508
Re: Mass of electrons
Because other units are based on kg, like joule(J) for energy and watt(W) for power. Joule is kg⋅m2⋅s−2, and watt is kg⋅m2⋅s−3. Using kg saves us from changing unit of mass during calculation.
- Mon Oct 15, 2018 12:23 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Negative sign in front of Bohr Frequency Condition
- Replies: 4
- Views: 471
Re: Negative sign in front of Bohr Frequency Condition
The energy is still positive. It's just a convention to set the energy of free electrons as 0, which makes electrons in atoms have negative values when the formula you mentioned is applied.
- Sun Oct 14, 2018 2:03 pm
- Forum: Significant Figures
- Topic: When to round the answers to significant figures
- Replies: 11
- Views: 2663
When to round the answers to significant figures
Do we round the answer in every step of the calculation, or do we just round the final answer to the number of significant figures? Because when I round the answer in every step, my final answers are often different from those given by the textbook.
- Sun Oct 14, 2018 1:14 pm
- Forum: Properties of Electrons
- Topic: Photoelectric Experiment
- Replies: 9
- Views: 551
Re: Photoelectric Experiment
maybe the larger number of photons means more energy in total
- Wed Oct 10, 2018 10:25 pm
- Forum: SI Units, Unit Conversions
- Topic: State of the molecules
- Replies: 4
- Views: 449
Re: State of the molecules
My TA also said that we don't need to worry about states for now
- Tue Oct 09, 2018 8:48 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Class Website Password
- Replies: 2
- Views: 322
Re: Class Website Password
Josephine Chan 1B wrote:Im not sure about 14b but for 14a, dr lavelle sent an email out about the class website with the password. So try checking the emails you got from the class email.
Thanks a lot!
- Tue Oct 09, 2018 8:33 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Class Website Password
- Replies: 2
- Views: 322
Class Website Password
Does anyone know the password to the 14B class website?
- Tue Oct 09, 2018 8:05 pm
- Forum: SI Units, Unit Conversions
- Topic: Conversions
- Replies: 2
- Views: 339
Re: Conversions
tonnes?
- Tue Oct 09, 2018 7:47 pm
- Forum: Balancing Chemical Reactions
- Topic: Percent Yield
- Replies: 5
- Views: 523
Re: Percent Yield
I think so. Percent yield is in some questions in Fundamentals.
- Tue Oct 02, 2018 1:04 pm
- Forum: Significant Figures
- Topic: Mass Percent Composition
- Replies: 4
- Views: 2695
Re: Mass Percent Composition
Should use significant figures instead of decimal places because finding the mass percent composition uses division. The number of significant figures in your answer should be the same as that of the value with the least significant figures that you use in the calculation.
- Tue Oct 02, 2018 12:58 pm
- Forum: Empirical & Molecular Formulas
- Topic: Empirical Formula Calculations
- Replies: 2
- Views: 363
Re: Empirical Formula Calculations
You can round the number. When calculating the empirical formula you often get numbers that are really close to whole numbers like 3.997 or 2.003. But if you get something like 6.667, consider multiplying it by a factor that gets you close to a whole number, like 3 in this case.
- Tue Oct 02, 2018 12:45 pm
- Forum: Administrative Questions and Class Announcements
- Topic: 'extra credit' in Sapling Learning
- Replies: 7
- Views: 1346
Re: 'extra credit' in Sapling Learning
hey, where did you find the quizzes, on the class website?
- Sun Sep 30, 2018 3:03 pm
- Forum: SI Units, Unit Conversions
- Topic: How does grading for discussion posts work?
- Replies: 80
- Views: 8581
Re: How does grading for discussion posts work?
Does anyone know when the grading for discussion posts starts, week 1?

