Search found 60 matches
- Sun Mar 17, 2019 8:27 am
- Forum: General Science Questions
- Topic: Midterm #7
- Replies: 1
- Views: 534
Re: Midterm #7
The combustion reaction is 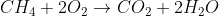 . Combustion reactions are typically writing as the organic molecule and oxygen as the reactants and water and carbon domioxide as the products. Combustion is typically an exothermic reaction.
. Combustion reactions are typically writing as the organic molecule and oxygen as the reactants and water and carbon domioxide as the products. Combustion is typically an exothermic reaction.
- Sun Mar 17, 2019 8:21 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Isothermal, Isobaric, Adiabatic
- Replies: 2
- Views: 621
Re: Isothermal, Isobaric, Adiabatic
I would know them just in case. Adiabatic means q=0, isothermal means constant temperature and isobaric means constant pressure. It’s also helpful to know that isochoric means constant volume.
- Sun Mar 17, 2019 8:04 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Approximations
- Replies: 1
- Views: 451
Re: Approximations
I think we have to decide on our own. If the K value is less than 10^-3 than I would assume to use the approximation. However, the safe bet is to use the quadratic formula.
- Thu Mar 07, 2019 7:02 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: kinetics vs thermodynamics
- Replies: 3
- Views: 420
Re: kinetics vs thermodynamics
While thermodynamics tells us why a chemical reaction occurs, kinetics tells us how a reaction occurs. The quantity related to kinetics is the rate constant and the thermodynamic quantity is correlated to delta G as it is the free energy associated given off during a a chemical reaction.
- Thu Mar 07, 2019 6:58 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Units of k
- Replies: 4
- Views: 490
Re: Units of k
To determine the units of the rate constant, a formula you could use to determine this is 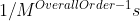 .
.
- Thu Mar 07, 2019 6:56 pm
- Forum: Zero Order Reactions
- Topic: Zero Order Reaction- how to identify
- Replies: 2
- Views: 344
Re: Zero Order Reaction- how to identify
Yes. For a zero-order reaction, the rate of the reaction is independent of reactant. The integrated rate law will help us determine is the reaction is zero-order is the plot of time (x-axis) vs. concentration of the reactant (y-axis) gives us a negative linear regression.
- Thu Feb 28, 2019 3:18 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Adding Standard Potentials
- Replies: 1
- Views: 308
Re: Adding Standard Potentials
Cell potential is an intensive property and is not a state function. Thus, we cannot just simply add the values together and apply a method similar to that of Hess's Law for cell potential. Therefore, since, delta G is a state function, we can use the equation \Delta G=-nFE to find the change in Gib...
- Thu Feb 28, 2019 3:14 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Potential Difference Conceptually
- Replies: 1
- Views: 198
Re: Potential Difference Conceptually
The cell potential is the potential difference associated with a galvanic cell that is working reversibly. A cell works reversibly when its pushing power is balanced against an external matching source of potential difference. The formal term for "voltage" os "potential difference,&qu...
- Thu Feb 28, 2019 3:06 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Finding n
- Replies: 12
- Views: 1128
Re: Finding n
Yes. For 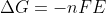 , n is the number of electrons that are transferred in the reaction
, n is the number of electrons that are transferred in the reaction
- Mon Feb 18, 2019 3:45 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Meaning of subscript r
- Replies: 3
- Views: 2538
Re: Meaning of subscript r
The subscript r is an abbreviation of "reaction." Thus delta H "r" would imply the change in enthalpy of the reaction and delta S "r" would signify the entropy change of the reaction.
- Mon Feb 18, 2019 3:43 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Pressure Change
- Replies: 4
- Views: 475
Re: Pressure Change
The change in entropy formula has final over the initial in that property because we are observing a change from an initial state to a final state. Pressure and volume are inversely proportional due to Boyle's Law and thus, pressure is an exception where it would be initial pressure over final press...
- Mon Feb 18, 2019 3:40 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Entropy change: V, P, T
- Replies: 4
- Views: 2951
Re: Entropy change: V, P, T
The higher the temperature, the more thermal energy the system has and thus, the system has more ways to distribute that energy. The more ways there are to distribute energy, the higher the entropy. The larger the volume, the more ways to distribute the molecules in that volume. The more ways there ...
- Sat Feb 16, 2019 10:12 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Max work
- Replies: 1
- Views: 230
Re: Max work
Delta G is equal to max work under constant temperature and pressure. G is defined as the maximum non-expansion work under constant T and P.
- Fri Feb 15, 2019 4:36 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: deltaS=qrev/T
- Replies: 1
- Views: 252
Re: deltaS=qrev/T
Not necessarily. Since entropy is a state function, finding the change in entropy irreversibly is the same as the change in entropy for a reversible reaction. Therefore, the change in entropy for a irreversible reaction is not immediately the negative of the reversible reaction.
- Tue Feb 12, 2019 12:09 pm
- Forum: Administrative Questions and Class Announcements
- Topic: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
- Replies: 179
- Views: 23962
Re: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
Can someone explain how to get the answer for question #6 part d?
- Sat Feb 09, 2019 1:00 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Standard Molar Entropy
- Replies: 1
- Views: 533
Re: Standard Molar Entropy
Due to the third law of thermodynamics, all substances have an absolute amount of entropy. Standard molar entropy is the total (minimal) amount of entropy that one mole of a substance gains as it is brought from 0 K to the standard conditions and thus, it is always positive (greater than zero) becau...
- Sat Feb 09, 2019 12:42 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Midterm
- Replies: 1
- Views: 263
Re: Midterm
In the sixth edition, the Gibbs Free Energy Problems are 9.51 through 9.68.
- Sat Feb 09, 2019 12:35 pm
- Forum: Calculating Work of Expansion
- Topic: Derivatives and Integrals
- Replies: 5
- Views: 661
Re: Derivatives and Integrals
I don’t think we need to use derivatives and integrals on the midterm but don’t quote me on this. I think we derived these equations in lecture/notes to understand where these variables and equations come from.
- Fri Feb 01, 2019 1:47 pm
- Forum: Phase Changes & Related Calculations
- Topic: PΔV of Solids and Liquids
- Replies: 4
- Views: 475
Re: PΔV of Solids and Liquids
Reactions that have a constant P and involves a solid and/or liquid will result in P V as insignificant. Because the volume of the reactants equals the volume of the products, there is no expansion work.
V as insignificant. Because the volume of the reactants equals the volume of the products, there is no expansion work.
- Fri Feb 01, 2019 1:43 pm
- Forum: Phase Changes & Related Calculations
- Topic: U and its relations to work
- Replies: 8
- Views: 838
Re: U and its relations to work
U is a state property and is the internal energy of a system. Changes in internal energy are a function of work. The energy of a closed system can be changed by heating/cooling or compression/expansion. \Delta U=q + w. If \Delta V is zero (constant volume), delta U= q_{v} . If \Delta P=0 (constant p...
- Fri Feb 01, 2019 1:27 pm
- Forum: Calculating Work of Expansion
- Topic: Maximum expansion work?
- Replies: 2
- Views: 535
Re: Maximum expansion work?
The reversible expansion does the maximum amount of work because the gas is pushing against the maximum possible external pressure.
- Fri Jan 25, 2019 12:05 am
- Forum: Phase Changes & Related Calculations
- Topic: Irreversible and Reversible process
- Replies: 2
- Views: 264
Re: Irreversible and Reversible process
A reversible process is a process that can be reversed in order to obtain the initial state of a system. There is also an equilibrium between the initial state and the final state of the system. An irreversible process is a thermodynamic process that cannot be reversed in order to obtain the initial...
- Thu Jan 24, 2019 11:50 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Delta H
- Replies: 4
- Views: 441
Re: Delta H
When delta H is positive, the positive sign indicates that the reaction is endothermic, meaning that it requires heat.
- Thu Jan 24, 2019 11:49 pm
- Forum: Phase Changes & Related Calculations
- Topic: State functions
- Replies: 4
- Views: 1030
Re: State functions
A state function depends only on the current state of the system, any change inits value is independent of how the change in state was brought about. Heat is not a state function because the energy transferred as heat during a change in the state of system depends on how the change is brought about....
- Wed Jan 16, 2019 12:50 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 7th Edition Question 6A.21
- Replies: 1
- Views: 221
7th Edition Question 6A.21
The value of K_{w} for water at body temperature (37 degrees Celsius) is 2.1x10^{-14} . How would you calculate the concentration of the hydronium ion and the hydroxide ion given only this information? Would you assume that the concentration of the hydronium ion is equivalent to the concentration to...
- Wed Jan 16, 2019 10:44 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ICE table
- Replies: 2
- Views: 499
Re: ICE table
You would typically use an ICE table when the reaction involves a weak acid or weak base. You would use molar ratios when the reaction involves a strong acid or strong base as these types of reactants completely dissociate. Weak acids and weak bases do not completely dissociate and thus, we would ha...
- Wed Jan 16, 2019 10:39 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Le Chatelier's Principle and Temperature Changes
- Replies: 3
- Views: 229
Re: Le Chatelier's Principle and Temperature Changes
When there is an increase in temperature of the system, Le Chatelier’s Principle states that the reaction will shift away from the heat and the energy of the exothermic reaction is favored. On the other hand, a decrease in temperature shifts the reaction toward the heat and the energy of the endothe...
- Tue Jan 08, 2019 2:07 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Concentrations
- Replies: 5
- Views: 457
Re: Concentrations
In regards to amount/number of moles, I like to visualize Le Chatelier's Principle like a seesaw. Let's say the left of the seesaw are the reactants and the right of the seesaw are the products. Increasing the amount of reactant leaves the seesaw tilted, having more weight on the left side. In order...
- Tue Jan 08, 2019 1:49 pm
- Forum: Ideal Gases
- Topic: Pressure units
- Replies: 4
- Views: 517
Re: Pressure units
You would commonly use torr or atmosphere as the units for partial pressure. I would stick with atmospheres to be safe. A bar is atmospheric pressure at sea level and equals 100 kilopascals.
- Tue Jan 08, 2019 1:45 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Question 5G.5 (7th Edition) Part C)
- Replies: 2
- Views: 283
Question 5G.5 (7th Edition) Part C)
Can someone please explain and clarify the following question: The following flasks (picture not included) show the dissociation of a diatomic molecule, 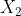 , over time.
, over time.
C)Assuming that the initial pressure of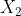 was 0.10 bar, calculate the value of K for the decomposition.
was 0.10 bar, calculate the value of K for the decomposition.
C)Assuming that the initial pressure of
- Mon Dec 03, 2018 9:27 am
- Forum: Bronsted Acids & Bases
- Topic: Ka Formula
- Replies: 6
- Views: 939
Re: Ka Formula
I would know this formula and how to apply it just in case. /[HA])) .
. 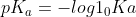
- Mon Dec 03, 2018 9:16 am
- Forum: Bronsted Acids & Bases
- Topic: Calculating pH
- Replies: 2
- Views: 309
Re: Calculating pH
If given the molarity of the strong acid solution, you would take the -log[H+] to calculate the pH. Since it is a strong acid, it completely dissociates and the molarity of the strong acid is equivalent to the concentration of H+ ions.
- Mon Dec 03, 2018 9:12 am
- Forum: Bronsted Acids & Bases
- Topic: Bronsted v Lewis
- Replies: 3
- Views: 415
Re: Bronsted v Lewis
All Bronsted-Lowry bases must have a lone pair to accept a hydrogen, and all Lewis bases have lone pairs. However, Lewis bases may act as nucleophiles (a chemical species that donates an electron pair to form a chemical bond in a reaction) for any number of atoms and not just for H.
- Fri Nov 30, 2018 9:33 am
- Forum: Hybridization
- Topic: Hybridization
- Replies: 2
- Views: 349
Re: Hybridization
Hybridization is the mixing of two non equivalent atomic orbitals. The result of hybridization is the hybrid orbital. Different types and numbers of atomic orbitals are participating in making hybrid orbitals. Different atomic orbitals have different shapes and number of electrons. But all the hybri...
- Fri Nov 30, 2018 9:29 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Oxidation numbers
- Replies: 1
- Views: 245
Re: Oxidation numbers
Here are some basic rules for assigning oxidation numbers: -The cation is written first in a formula, followed by the anion. -The oxidation number of a free element is always 0. -The oxidation number of a monoatomic ion equals the charge of the ion. -The usual oxidation number of hydrogen is +1. Exc...
- Fri Nov 30, 2018 9:24 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Transition Metals
- Replies: 4
- Views: 331
Re: Transition Metals
Electron transfers are essentially redox reactions, where the oxidation state of the reactant and product change as the process of an electron moves from one atom or molecule to another. Transition metals can exist in more than one oxidation state and thus electron transfer reactions commonly involv...
- Thu Nov 22, 2018 3:36 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 7th Edition Question 2E. 29
- Replies: 2
- Views: 119
7th Edition Question 2E. 29
Part b) of question 2E. 29), is asking us to identify which of the three isomers of dichlorobenzene has the largest dipole moment. Can someone explain to me why the first structure has the largest dipole moment in comparison to the second isomer?
- Thu Nov 22, 2018 12:53 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Does formal charge apply to the VSEPR Model?
- Replies: 6
- Views: 1789
Does formal charge apply to the VSEPR Model?
When predicting the molecular shape, I understand that the first step is to draw the lewis structure for the molecule; however do we account for formal charges in this situation? In other words, do we use the most stable form of the lewis structure to determine the shape?
- Thu Nov 22, 2018 10:11 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: molecular vs electron geometry
- Replies: 2
- Views: 167
Re: molecular vs electron geometry
Electron geometry teaches us about the arrangement of different electron groups and we consider both the lone electron pairs and bond electron pairs. Through electron geometry we get the spatial arrangement of lone pairs and bond in the molecule. Molecular geometry helps us understand the entire ato...
- Wed Nov 14, 2018 9:23 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: MgO vs BaO
- Replies: 5
- Views: 3549
Re: MgO vs BaO
BaO is more soluble in water than MgO because its atomic size gives the molecule a more iconic nature and less lattice energy. The MgO molecule has a cation with a smaller radius, indicating that there is strong attraction between the cation and anion in the lattice.
- Tue Nov 13, 2018 12:05 am
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Hybrid vs. Molecular Orbitals
- Replies: 3
- Views: 2578
Hybrid vs. Molecular Orbitals
What are the differences between a hybrid and molecular orbital?
- Tue Nov 13, 2018 12:00 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Axial vs. Equatorial Lone Pairs
- Replies: 2
- Views: 258
Axial vs. Equatorial Lone Pairs
Can someone distinguish the difference between axial lone pairs and equatorial lone pairs and explain how they contribute to molecular structure?
- Thu Nov 08, 2018 6:23 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Polarizability and Dispersion Forces
- Replies: 2
- Views: 502
Re: Polarizability and Dispersion Forces
Dispersion forces tend to be stronger between molecules that are easily polarized and weaker between molecules that are not easily polarized.
- Thu Nov 08, 2018 5:57 pm
- Forum: Sigma & Pi Bonds
- Topic: sigma/pi bonds and bond order
- Replies: 2
- Views: 467
Re: sigma/pi bonds and bond order
A single bind is a sigma bond. A sigma bond is cylindrically symmetrical with no nodal planes containing to the internuclear axis. All single covalent bonds are sigma bonds. A pi bond has a single nodal plane containing to the internuclear axis. A double bond is typically considered a pi bond.
- Thu Nov 08, 2018 5:44 pm
- Forum: Dipole Moments
- Topic: Induced Dipole
- Replies: 4
- Views: 350
Re: Induced Dipole
The dipole-induced-dipole interaction is when a polar molecule interacts with a non polar molecule. This interaction arises from the ability of one molecule to induce a dipole moment in the other. However, in this case, the molecule that induces the dipole moment has a permanent dipole moment. The p...
- Thu Nov 01, 2018 12:07 pm
- Forum: Resonance Structures
- Topic: Definition of resonance
- Replies: 7
- Views: 907
Re: Definition of resonance
Resonance is the blending of structures; some lewis structures have multiple bonds in different locations and a resonance hybrid is a structure of the contributing Lewis structures.
- Thu Nov 01, 2018 10:52 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Difference Between Atomic or Molecular Spectroscopy
- Replies: 2
- Views: 15524
Re: Difference Between Atomic or Molecular Spectroscopy
Atomic and Molecular Spectroscopy present more similarities than differences. Electrons in atoms can be excited to higher energy states and electrons in molecules can also be excited to high energy states. As a result, both atoms and molecules give rise to spectra.
- Thu Nov 01, 2018 10:46 am
- Forum: Formal Charge and Oxidation Numbers
- Topic: Lowest Energy Lewis Structure: N2O
- Replies: 2
- Views: 4299
Re: Lowest Energy Lewis Structure: N2O
The most stable resonance nitrous oxide lewis structure would have the formal charges of +1 for N, -1 for O, and 0 for N. The lowest energy/best structure would have the overall formal charge minimized; in this case, the resonance structure of 0 for N, +1 for O, and +1 for N has an overall formal ch...
- Fri Oct 26, 2018 1:45 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Ground state vs. excited state
- Replies: 5
- Views: 784
Re: Ground state vs. excited state
Cations are positively charged ions; they are generally smaller than their parent atoms. On the other hand, anions are negatively charged ions; they are generally larger than their parent atoms.
- Fri Oct 26, 2018 1:40 am
- Forum: Trends in The Periodic Table
- Topic: Covalent Radius
- Replies: 4
- Views: 413
Re: Covalent Radius
Think of a covalent radius as a subdivision of atomic radii. The atomic radius of an element is defined as half the distance between the centers of neighboring atoms. The covalent radius of an element, specifically nonmetals and metalloids, is half the distance between the nuclei of atoms joined by ...
- Fri Oct 26, 2018 1:34 am
- Forum: DeBroglie Equation
- Topic: momentum of light
- Replies: 2
- Views: 319
Re: momentum of light
De Broglie proposed that all particles should be regarded as having wavelike properties. He went on to suggest that the wavelength associated with the "matter wave" is inversely proportional to the particles mass and speed. Photons are indeed massless; however, you must take under consider...
- Tue Oct 16, 2018 2:12 pm
- Forum: Einstein Equation
- Topic: Energy
- Replies: 2
- Views: 310
Re: Energy
The energy per photon is E=hv. h is Planck's constant (6.626 x 10^-34) and v is the frequency. One photon interacts with one electron. Each photon must have enough energy to eject one electron. Increasing light intensity increases the number of photons. If the energy per photon is greater or equal t...
- Tue Oct 16, 2018 11:34 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Heisenberg's Indeterminacy Equation
- Replies: 3
- Views: 263
Re: Heisenberg's Indeterminacy Equation
- Tue Oct 16, 2018 10:38 am
- Forum: Properties of Light
- Topic: Week 3 HW
- Replies: 5
- Views: 548
Re: Week 3 HW
Yes. For the week three homework, turn in another seven problems from the quantum world section. Chapter 1 in the book concentrates on what we are learning in class.
- Thu Oct 11, 2018 12:07 pm
- Forum: Administrative Questions and Class Announcements
- Topic: So can we do problems from Review of Chemistry for week 2 homework?
- Replies: 4
- Views: 298
Re: So can we do problems from Review of Chemistry for week 2 homework?
No, the homework due in your discussion for week two should be the quantum world problem set. Choose seven problems that you can do based on what we already learned in class.
- Thu Oct 11, 2018 12:03 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Balmer/Lyman... Series
- Replies: 4
- Views: 357
Re: Balmer/Lyman... Series
Consider memorizing the Lyman (n=1), Balmer (n=2), Paschen (n=3), and Brackett (n=4) series.
- Thu Oct 11, 2018 11:59 am
- Forum: Properties of Electrons
- Topic: Atomic spectra
- Replies: 5
- Views: 455
Re: Atomic spectra
Spectroscopic analysis of light given off by excited atoms shows only photons of particular energy (v) are given off. The energy of the photon coming in corresponds to the difference in energy levels. You use the results of the light experiment to figure out what the structure is of your sample. Thu...
- Fri Oct 05, 2018 9:42 am
- Forum: Limiting Reactant Calculations
- Topic: When to use H vs H2
- Replies: 7
- Views: 1105
Re: When to use H vs H2
In the case in which 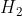 is presented as a diatomic molecule, the molar mass of H would be multiplied by two. Therefore, the molar mass of
is presented as a diatomic molecule, the molar mass of H would be multiplied by two. Therefore, the molar mass of 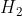 is 2.016
is 2.016 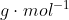 .
.
- Fri Oct 05, 2018 9:20 am
- Forum: Balancing Chemical Reactions
- Topic: Post-Module Assessment typo?? - Balancing Chemical Reactions
- Replies: 3
- Views: 430
Re: Post-Module Assessment typo?? - Balancing Chemical Reactions
There is no typo. The correct answer is D) +2O{_{2}}(g)\rightarrow CO{_{2}}(g)+2H{_{2}}O(g)) . Answer choice D is the best answer choice for the balanced equation as the ratio of moles (the stoichiometric coefficients) are presented in the lowest terms.
. Answer choice D is the best answer choice for the balanced equation as the ratio of moles (the stoichiometric coefficients) are presented in the lowest terms.
- Fri Oct 05, 2018 9:03 am
- Forum: Limiting Reactant Calculations
- Topic: Excess Reactant Calculation
- Replies: 2
- Views: 303
Re: Excess Reactant Calculation
After balancing the equation for the reaction, calculate the molar masses for each of the reactants and products. Then convert the known masses of the reactant and products to moles. In order to identify the limiting and excess reagents, compare the calculated moles to required moles to determine th...

