Search found 66 matches
- Sun Mar 17, 2019 6:46 pm
- Forum: Calculating Work of Expansion
- Topic: Conversion Units for Work Expansion
- Replies: 1
- Views: 455
Re: Conversion Units for Work Expansion
Work is usually reported in Joules, so the 101.325 J/L*atm would most likely be used when you are converting the value gained from an irreversible expansion. The units of the value gained from using the equation to calculate the work done from a reversible expansion is already in the units of joules...
- Fri Mar 15, 2019 11:58 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Textbook Question 7D.5 (7th edition)
- Replies: 1
- Views: 268
Re: Textbook Question 7D.5 (7th edition)
You would use a derived version of the Arrhenius equation: ln(k2/k1)= (Ea/R) ((1/T1)-(1/T2)). Solving for k2, since you are given the rest of the information needed for the rest of the equation.
- Fri Mar 15, 2019 11:47 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Cell Work
- Replies: 4
- Views: 656
Re: Cell Work
The maximum work that a cell can do is equal to the change in Gibbs free energy of the cell.
- Fri Mar 15, 2019 9:36 pm
- Forum: Second Order Reactions
- Topic: Non-integer orders
- Replies: 7
- Views: 1172
Re: Non-integer orders
Lavelle says that it is not likely that we would get a non-integer order, since that is not common in natural reactions. You might get one that is a few decimal points off ( 1.97), thus you would just assume this is from experimental errors and round to 2.
- Fri Mar 08, 2019 12:53 am
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Unique Average Rate
- Replies: 5
- Views: 588
Re: Unique Average Rate
Yes, the unique average rate of reaction for each product/reactant is the same, as long as you know the change concentrations of each substance, the corresponding coefficient, and the change in time.
- Fri Mar 08, 2019 12:49 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: reaction orders
- Replies: 2
- Views: 280
Re: reaction orders
They just represents how much they affect the reaction rate. Thus zeroth order has no effect, first has and effect proportional to the change to the reactant, and second has an affect proportional to the change raised to the second power. It is a number that relates the rate of the chemical reaction...
- Thu Mar 07, 2019 4:22 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Unique Average Rate
- Replies: 5
- Views: 588
Re: Unique Average Rate
The unique average rate of a reaction is just the rate of a reaction but put in terms that the rate of change of a specific reactant or product of the reaction are all equal to each other. Dividing by their coefficients and making sure to make the rate of change of the reactants negative, each indiv...
- Thu Mar 07, 2019 4:18 pm
- Forum: Zero Order Reactions
- Topic: Concentration independent of the rate
- Replies: 4
- Views: 556
Re: Concentration independent of the rate
Do you mean the rate of a reaction is independent of the concentration of the reactant? If so, just look at the table and see if that specific reactant concentration is changed ( for example it is doubled), then you check the rate of the reaction. If the rate of the reaction did not change due to th...
- Thu Feb 28, 2019 3:28 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Understanding cell diagrams
- Replies: 2
- Views: 361
Re: Understanding cell diagrams
It is used if one(or both) if the oxidizing(or reducing ) agents are not conductors. Thus, if they are not a metal or if they aqueous ions, before and after the reaction, you need an inert metal such at Pt, to be the electrode and transfer electrons. In the cell diagram, you would just add a vertica...
- Thu Feb 28, 2019 3:24 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: H+, OH-, water
- Replies: 9
- Views: 936
Re: H+, OH-, water
I would think that you would need to include the H+ and OH- in the cell diagrams, but I dont think water is included.
- Thu Feb 28, 2019 3:19 pm
- Forum: Balancing Redox Reactions
- Topic: Writing half reactions
- Replies: 7
- Views: 649
Re: Writing half reactions
The H+ will be with the Cr207 because the h20 will be included with this half reaction to balance the oxygens, and the H+ will be needed the balance the hydrogens from the h20.
- Thu Feb 21, 2019 11:29 pm
- Forum: Balancing Redox Reactions
- Topic: Balancing Redox Rxns
- Replies: 2
- Views: 338
Re: Balancing Redox Rxns
How would one figure out the oxidation numbers for certain elements in a reaction?
- Thu Feb 21, 2019 11:22 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: nerst equation
- Replies: 3
- Views: 329
Re: nerst equation
What is the difference between cell potential and standard cell potential?
- Thu Feb 21, 2019 11:21 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: nerst equation
- Replies: 3
- Views: 329
nerst equation
In what situation would you know to use the nerst equation?
- Thu Feb 21, 2019 11:14 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode and Cathode
- Replies: 4
- Views: 481
Re: Anode and Cathode
Oxidation occurs at the anode and reduction occurs at the cathode
- Fri Feb 15, 2019 2:37 am
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: KbNa
- Replies: 5
- Views: 758
Re: KbNa
The Boltzmann constants is in terms of per particle. Thus to get to the Boltzmann constants' units of J/K, you would just have to divide the gas constant (8.315 J/(mol*K)) by avagadro's number to get rid of the moles and get it into the per particle terms.
- Fri Feb 15, 2019 2:30 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: using gas constant R
- Replies: 4
- Views: 526
Re: using gas constant R
I dont think R is ever used as a C value in the entropy equation as temperature changes. The C value also changes depending on the shape od the molecule. Th R value by itself would mostly be used in the work equation for reversible work.
- Fri Feb 15, 2019 2:26 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Calculating Delta S with a change in temperature
- Replies: 5
- Views: 588
Re: Calculating Delta S with a change in temperature
Also take into account the shape of the molecule because whether it is monatomic, linear, or non-linear changes the C value in both constant pressure and volume.
- Thu Feb 07, 2019 4:04 pm
- Forum: Calculating Work of Expansion
- Topic: Gas Constant
- Replies: 3
- Views: 449
Re: Gas Constant
The 8.314 J*mol^-1 *K^-1 would be the R value that you need to calculate the w in that equation. If you need to convert units, 101.325 J= 1L*atm. Thus R also equals 0.082057 L*atm* mol^-1 *K^-1, which is the R value used In the ideal gas law.
- Thu Feb 07, 2019 3:55 pm
- Forum: Calculating Work of Expansion
- Topic: Different Work Equations
- Replies: 2
- Views: 278
Re: Different Work Equations
It should let you know in the problem. If they are asking for the work done in an isothermal expansion (constant temperature), you probably have to solve for the reversible pathway, since the work the system is doing if being provided energy by some outside source, such as a heat reservoir. If the q...
- Thu Feb 07, 2019 3:51 pm
- Forum: Calculating Work of Expansion
- Topic: Reversible vs. Irreversible
- Replies: 2
- Views: 286
Re: Reversible vs. Irreversible
Almost all real world reactions that occur are irreversible, since the change in volume happens spontaneously. A reversible reaction is purely hypothetical, since it cannot happen in the real world. However, in the case of this class, the problem will probably tell you if they want you to calculate ...
- Fri Feb 01, 2019 1:11 am
- Forum: Calculating Work of Expansion
- Topic: Equations for w
- Replies: 5
- Views: 487
Re: Equations for w
The integral equation is used when calculating reversible expansion done by a gas, since in this case it is set up so that the change in pressure occurs in small increments till it reaches the volume it should be at in equilibrium. In reversible expansion, the external and internal pressures are nea...
- Fri Feb 01, 2019 1:05 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: q=-q
- Replies: 4
- Views: 2101
Re: q=-q
Heat flows from hot to cold and because the temperature of the two are now the same and will remain constant, heat will not be transferred between them
- Fri Feb 01, 2019 1:02 am
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Reversible and isothermal
- Replies: 2
- Views: 374
Re: Reversible and isothermal
If a reaction is reversible, it just means that if either the external or the internal pressure are very close to equal and that if either of them is changed slightly, the volume of the container would change. Thus the change in pressure of the reaction is occurring in very small units as it approac...
- Thu Jan 24, 2019 5:20 pm
- Forum: Ideal Gases
- Topic: Ice Table with quadratic equation on bottom
- Replies: 3
- Views: 335
Re: Ice Table with quadratic equation on bottom
This seems possible. You would just have to multiply the denominator out( for example (1-x)(2-x) = 2-3x+x^2). Then multiply the denominator with the K value. Last just subtract the x value from both sides to get the equation you need to plug into the quadratic equation.
- Thu Jan 24, 2019 5:15 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Strong vs. Weak Acids
- Replies: 3
- Views: 406
Re: Strong vs. Weak Acids
Im not to sure about the exact concentration of hydronium or hydroxide, but strong acids/bases have pKa/pKb values that are greater than 10^3, and at that amount you can be sure that they fully dissociate.
- Thu Jan 24, 2019 5:00 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Le Chateliers Priniciple
- Replies: 9
- Views: 936
Re: Le Chateliers Priniciple
Yes, in short it will cause the reaction to shift towards the side with the least amount of total molecules.
- Fri Jan 18, 2019 2:05 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Understanding Q
- Replies: 3
- Views: 320
Re: Understanding Q
The quotient (Q), is simply the ratio of products:reactants at that point in the reaction. Eventually Q will have to approach K and thus we can make assumptions about how the reaction will proceed using the Q.
- Fri Jan 18, 2019 2:02 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Weak Acids and Bases
- Replies: 7
- Views: 828
Re: Weak Acids and Bases
Since there are so many more weak acid/bases than there are strong ones, I would suggest memorizing the strong ones instead. Last quarter, Lavelle gave us a run through of how you can easily identify the strong acids. Additionally, most likely the question will at least give you a Kb or a Ka, and fr...
- Fri Jan 18, 2019 2:00 pm
- Forum: Ideal Gases
- Topic: Pressure in terms of mols
- Replies: 3
- Views: 462
Re: Pressure in terms of mols
In part, yes. The less moles there are, the less molecules you have colliding with the surface of the container and thus applying less pressure. Also consider the equation PV= nRT, as the number of moles decreases, the pressure has to decrease.
- Thu Jan 17, 2019 11:59 pm
- Forum: Ideal Gases
- Topic: Q and K Graph Explanation
- Replies: 2
- Views: 270
Re: Q and K Graph Explanation
I don't remember a bar graph relating Q and K. However, just keep in mind that if Q<K, the products are still being produced at the cost of the reactants in the forward reaction. and if Q>K, the products are still being used to create the reactants in the reverse reaction. The only bar graph that I ...
- Sat Jan 12, 2019 10:03 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Understanding Q
- Replies: 3
- Views: 320
Re: Understanding Q
Recall that you solve for K=(products)/(reactants). Thus when Q<K, to increase Q and get the reaction to equilibrium, you would have to increase the numerator(products) and decrease the denominator(reactants). That is why more products are formed. The same can be said for when Q>K, to decrease Q and...
- Sat Jan 12, 2019 9:58 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: 6th edition, 5J11
- Replies: 2
- Views: 159
Re: 6th edition, 5J11
Im not too sure, but considering the fact that diatomic halogens are more stable than the products, I would assume that the reaction of the decomposition is endothermic since you would have to put additional energy into the reaction to break the bond and make it go from a stable state to a less stab...
- Sat Jan 12, 2019 9:51 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: pressure and temperature
- Replies: 4
- Views: 205
Re: pressure and temperature
Changing pressure would result in a change in volume and since concentration= moles/volume, the concentration of the reactants and products would change respectively. Changing temperature would cause the concentration of either the reactants or products to change depending on if the forward/reverse ...
- Mon Dec 10, 2018 12:07 am
- Forum: Lewis Acids & Bases
- Topic: Lewis and Bronsted-Lowry Acids and Bases
- Replies: 1
- Views: 446
Re: Lewis and Bronsted-Lowry Acids and Bases
Lewis acids are those that accept e- pairs, while Lewis bases are those that donate e- pairs. Bronsted acids donate H+ (protons) while Bronsted bases accept H+. Thus the main difference between the two is more to do with what they are focusing on during the reaction, e- pairs for Lewis and H+ for Br...
- Mon Dec 10, 2018 12:01 am
- Forum: Lewis Structures
- Topic: drawing complex lewis structures
- Replies: 1
- Views: 630
Re: drawing complex lewis structures
My take on the process would be to just start by drawing out the molecule from left to right. The beginning is pretty simple, with a carbon in the center bonded to 3 hydrogens and 1 carbon. The COOH part is just to show that the hydrogen is attached to the oxygen rather than to the carbon. If it wer...
- Thu Nov 15, 2018 9:29 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Trigonal Pyramidal
- Replies: 10
- Views: 487
Re: Trigonal Pyramidal
The shape of the molecule with the lone pair doesn't make the molecule trigonal planar. The lone pair is still there and thus keeps the electronic shape tetrahedral, and its molecular shape trigonal pyramidal.
- Thu Nov 15, 2018 9:25 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Trigonal Pyramidal
- Replies: 10
- Views: 487
Re: Trigonal Pyramidal
The lone pair still plays a role in the shape of the molecule, thus making the electronic shape tetrahedral. Just picture a tetrahedral shape with the atom at the top missing, leaving a trigonal pyramidal shape, with bond angles that are slightly less than 109.5
- Thu Nov 15, 2018 9:20 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Trigonal Pyramidal
- Replies: 10
- Views: 487
Re: Trigonal Pyramidal
Its electronic shape would be that of a tetrahedral, while the molecular shape is that of trigonal pyramidal. Maybe you confused the two terms?
- Thu Nov 15, 2018 9:16 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Trigonal Pyramidal
- Replies: 10
- Views: 487
Re: Trigonal Pyramidal
It has 4 regions of electron density because lone pairs count as areas of electron density as well. A double or triple bond is still considered only one region of electron density. The bond angles are less than 109.5 because lone pairs have a stronger repulsion than bonded pairs.
- Thu Nov 15, 2018 9:13 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: T-shaped molecules
- Replies: 2
- Views: 275
Re: T-shaped molecules
I would think that they have very similar bond angles, ones that are <90 degrees due to the lone pairs. However the one with 3 lone pairs might have a slightly smaller angle. Although, I dont think we would need to know the specific angles for each, just that they are less than what you would expect...
- Thu Nov 15, 2018 9:02 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Linear Shape
- Replies: 5
- Views: 384
Re: Linear Shape
Yes, for example for if a central atom with 5 regions of electron density has 3 lone pairs, it will have a linear shape. The same can be said for a central atom with 6 regions of electron density that has 4 lone pairs.
- Thu Nov 15, 2018 12:37 am
- Forum: Octet Exceptions
- Topic: d orbital period 3
- Replies: 2
- Views: 1842
Re: d orbital period 3
It has access to its d orbital because it is in the 3p block, which is close enough to the 3d sub shell to use when making bonds. However, in its ground state and when it is not making any bonds, its 3d sub shell is not in use.
- Wed Nov 14, 2018 11:21 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Drawing structures
- Replies: 4
- Views: 385
Re: Drawing structures
Yes NH3 would have a shape of trigonal pyramidal, since the lone pair doesn't count as part of the shape. However, its electronic shape (shape considering all regions of electron density) would be tetrahedral.
- Thu Nov 08, 2018 10:17 pm
- Forum: Bond Lengths & Energies
- Topic: Dipole Moment Units
- Replies: 3
- Views: 411
Re: Dipole Moment Units
This measures the polarity of a bond within a molecule and is measured in coulomb-meters.
- Thu Nov 08, 2018 10:15 pm
- Forum: Dipole Moments
- Topic: Attractive Force
- Replies: 3
- Views: 212
Re: Attractive Force
Increasing the size of an atom increases the attractive forces because it increases the dispersion forces, or the uneven distribution of electrons in the electron cloud, and thus creates more dipole and induced dipole moments.
- Thu Nov 08, 2018 6:01 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizability and States of Matter
- Replies: 2
- Views: 556
Re: Polarizability and States of Matter
Molecules with high polarizability boil at higher temperatures because polarizability is how easy it is to move its electron cloud. Thus those with easily movable electron clouds are more likely to have more dispersion forces, which are the uneven dispersion of electron in the electron cloud, and th...
- Fri Nov 02, 2018 12:07 am
- Forum: Ionic & Covalent Bonds
- Topic: Valence electron
- Replies: 4
- Views: 332
Re: Valence electron
The valence electrons for the elements in the d block are always the amount of electron in the outermost shell. For example, for Mn: [Ar] 4s^2 3d^5. There are 2 valence electrons because the 4th energy level is the outer most orbital.
- Thu Nov 01, 2018 11:56 pm
- Forum: Ionic & Covalent Bonds
- Topic: Electron Configuration
- Replies: 3
- Views: 338
Re: Electron Configuration
The 4s orbital is written before the 3d orbital to show that it is of lower energy, since the farther the orbital you go, the more energy it has.
- Thu Nov 01, 2018 11:30 pm
- Forum: Resonance Structures
- Topic: 6th Edition 3.21
- Replies: 2
- Views: 311
Re: 6th Edition 3.21
Both would be correct to identify the ion. If you want to show where the electrons are more specifically, you should do the entire ground state configuration, which is probably the best way to answer the question.
- Fri Oct 26, 2018 12:28 am
- Forum: DeBroglie Equation
- Topic: Measurable wavelength properties
- Replies: 5
- Views: 581
Re: Measurable wavelength properties
I would think he just meant if the car had a wavelength that was big enough to notice and detect while moving. For example, when you did the calculation for the wavelength of a car with a mass of 1.5 * 10^8 kg moving at a speed of 27 m/s, the wavelength would be 1.64 *10^-38, which is really small a...
- Fri Oct 26, 2018 12:21 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Configuration and Unfilled Orbitals
- Replies: 2
- Views: 304
Re: Electron Configuration and Unfilled Orbitals
Yes the 6s orbital would begin to fill before the 5d orbital because it has a lower energy than that of the 5d orbital. Keep in mind that lower energy orbitals always fill up before higher orbitals. If you need more clarification: https://www.chemguide.co.uk/atoms/prope ... oblem.html
- Fri Oct 26, 2018 12:15 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: pauli exclusion
- Replies: 3
- Views: 403
Re: pauli exclusion
It basically means that there can only be two electrons in each orbital (state). For example, when drawing an aufbau diagram, in each orbital there are only two electrons, the one that spins up and the one that spins down.
- Fri Oct 26, 2018 12:12 am
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Conceptual question
- Replies: 3
- Views: 431
Re: Conceptual question
It plays into the fact that we are only talking about a particle with very little mass, such as that of an electron. When trying to measure the speed of a particle passing by we would count how long it takes to move from one position to another. However, when you do so you are messing up the origina...
- Fri Oct 26, 2018 12:06 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: atomic spectra Rydberg
- Replies: 3
- Views: 412
Re: atomic spectra Rydberg
You should use the smaller n value as the n1 value and the bigger one as the n2, because the n1 should be the initial state.
- Thu Oct 18, 2018 2:12 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Atomic Spectra Module #41
- Replies: 3
- Views: 1547
Re: Atomic Spectra Module #41
Its B. You know that an electron going from 5 to 1 would emit more energy than that from 4 to 2, since the energy emitted is proportional to the distance the electron falls. Energy is also inversely proportional to wavelength, with higher energy having smaller wavelengths. If you would want to see t...
- Thu Oct 18, 2018 1:14 pm
- Forum: Properties of Light
- Topic: Friday 10/5 Lecture
- Replies: 7
- Views: 669
Re: Friday 10/5 Lecture
keep in mind that v= velocity, and that 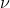 = frequency(curly v). But yes in that case v is velocity
= frequency(curly v). But yes in that case v is velocity
- Thu Oct 18, 2018 1:06 pm
- Forum: Properties of Electrons
- Topic: electrons
- Replies: 3
- Views: 251
Re: electrons
Right, id think the amount of electrons would play a role in the energy of the electron due to the repulsion. I would also think then that the element plays a role due to the amount of protons and neutrons, which keep the atom together and thus change the amount of energy needed to excite the electr...
- Thu Oct 18, 2018 12:49 pm
- Forum: Properties of Electrons
- Topic: Energy v. Kinetic Energy
- Replies: 3
- Views: 1595
Re: Energy v. Kinetic Energy
I think the equation for E=hc is only for light, so even though an electron has both waves and photon like properties, you won't get the same answer as if you used the de Broglie equation which is for particles.
- Thu Oct 11, 2018 10:46 am
- Forum: Properties of Electrons
- Topic: Exercise 1B 19
- Replies: 2
- Views: 436
Exercise 1B 19
The question states that "protons and neutrons have nearly the same mass. How different are their wavelengths? Calculate the wavelength of each particle when traveling at 2.75 * 10^5 m/s in a particle accelerator and report the difference as a percentage of the wavelength of the neutron." ...
- Thu Oct 11, 2018 10:36 am
- Forum: Properties of Light
- Topic: Memorizing wavelengths and frequencies
- Replies: 5
- Views: 1731
Re: Memorizing wavelengths and frequencies
I would assume so, since some of the homework problems include those types of questions. Just remember that visible light is between 400 to 700 nm, infrared is between 700 to 1000 nm, UV is 10 to 400 nm, and gamma rays are anything smaller than that. You could memorize the frequencies if you'd like,...
- Thu Oct 11, 2018 10:31 am
- Forum: Properties of Electrons
- Topic: Energy of Photons
- Replies: 1
- Views: 175
Energy of Photons
Hi,
I've seen some questions where they list the energy of the photon in keV. Is this similar to an electron volt (eV)? and how would you convert this to joules?
I've seen some questions where they list the energy of the photon in keV. Is this similar to an electron volt (eV)? and how would you convert this to joules?
- Thu Oct 04, 2018 9:16 pm
- Forum: Empirical & Molecular Formulas
- Topic: Question M.19 (Sixth Edition)
- Replies: 2
- Views: 355
Re: Question M.19 (Sixth Edition)
This is a combustion analysis question. I would start the question to trying to find the exact masses of carbon, hydrogen, nitrogen, and possibly oxygen too. Take the mass of each product and convert it to mass to the exact mass of that element produced. For example, with the carbon dioxide, divide ...
- Thu Oct 04, 2018 8:51 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Homework Question G5
- Replies: 3
- Views: 218
Re: Homework Question G5
The way I would approach this problem would to at first convert the mass of sodium carbonate to moles of the compound, and then divide this by .25 L to get the molarity of the solution (0.0796 M). Now for each question that they are asking for, start by converting the mmoles to moles by dividing by ...
- Thu Oct 04, 2018 8:31 pm
- Forum: Significant Figures
- Topic: Limiting Reactants
- Replies: 6
- Views: 627
Re: Limiting Reactants
The post above is correct, but there are multiple ways to approach these types of problems. The method I usually use is to take to given masses of the reactants and convert them to moles of reactants. Followed by using the mole ratio and converting that to the total moles of the product that can be ...
- Wed Oct 03, 2018 11:28 am
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Question E 15
- Replies: 2
- Views: 166
Question E 15
The question states that the molar mass of the metal hydroxide M(OH)2 is 74.10 g/mol and asks to calculate the molar mass of the sulfide of the metal. I'm assuming that the M is a variable for a certain metal with a +2 charge. Is the question simply asking for the molar mass of MS, by subtracting th...

